Mole to Mole Conversions Worksheet Answer Key
Are you a chemistry enthusiast looking to improve your understanding of mole to mole conversions? Look no further! In this blog post, we will provide you with a detailed answer key for the Mole to Mole Conversions Worksheet. This worksheet is designed for high school or college students who are studying chemistry and want to practice and reinforce their knowledge of converting between moles of different substances in chemical equations.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
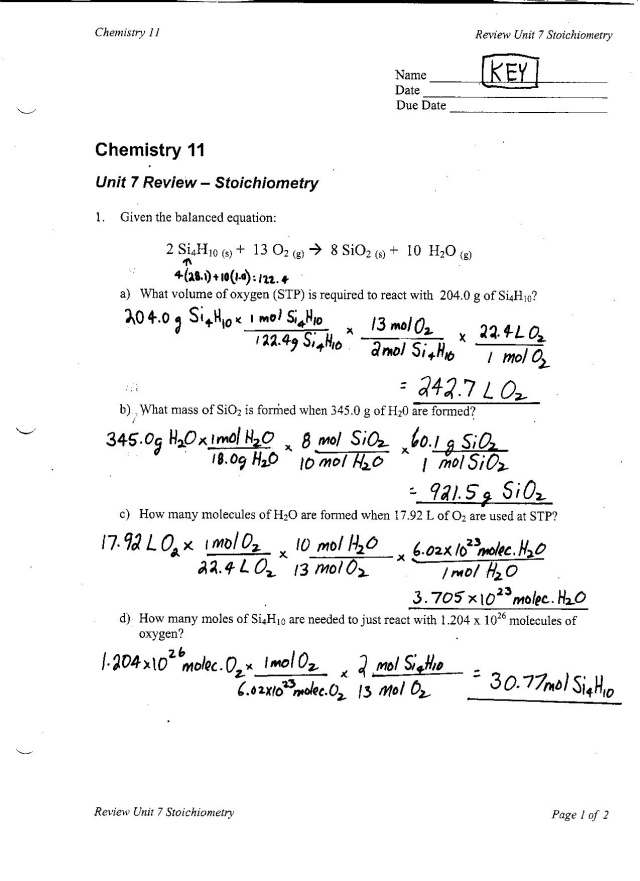
What is a mole-to-mole conversion?
A mole-to-mole conversion is a process in chemistry that involves converting the amount of one chemical species in moles to the amount of another chemical species in moles, based on the balanced chemical equation relating the two species. This conversion is important for determining the stoichiometry of a chemical reaction and for predicting the amounts of reactants and products involved in the reaction.
How do you determine the number of moles of a substance in a given chemical equation?
To determine the number of moles of a substance in a given chemical equation, you can use the coefficients of the balanced equation as the mole ratios. By comparing the mole ratios of the substance you are interested in to a substance with a known number of moles, you can calculate the number of moles of the substance in question. This method follows the principle of stoichiometry, which allows you to convert between the different quantities of substances involved in a chemical reaction.
How can you use a balanced chemical equation to convert moles of one substance to moles of another substance?
To use a balanced chemical equation to convert moles of one substance to moles of another substance, you must first identify the stoichiometry relationship between the two substances in the equation. This is done by comparing the coefficients of the two substances in the balanced chemical equation. Once you have determined this relationship, you can set up a conversion factor based on the coefficients to convert moles of one substance to moles of the other. By using this conversion factor, you can calculate the moles of the desired substance based on the given moles of the initial substance.
What is the relationship between moles and the coefficients in a balanced chemical equation?
The coefficients in a balanced chemical equation represent the ratio of moles of each reactant and product participating in the reaction. This means that for every balanced chemical equation, the coefficients can be used to determine the amounts of substances involved in the reaction in terms of moles. For example, if the coefficient of a substance is 2, it means that 2 moles of that substance are involved in the reaction. Therefore, the coefficients in a balanced chemical equation directly relate to the mole ratios of substances in the reaction.
How can you calculate the number of moles of a substance when given the mass?
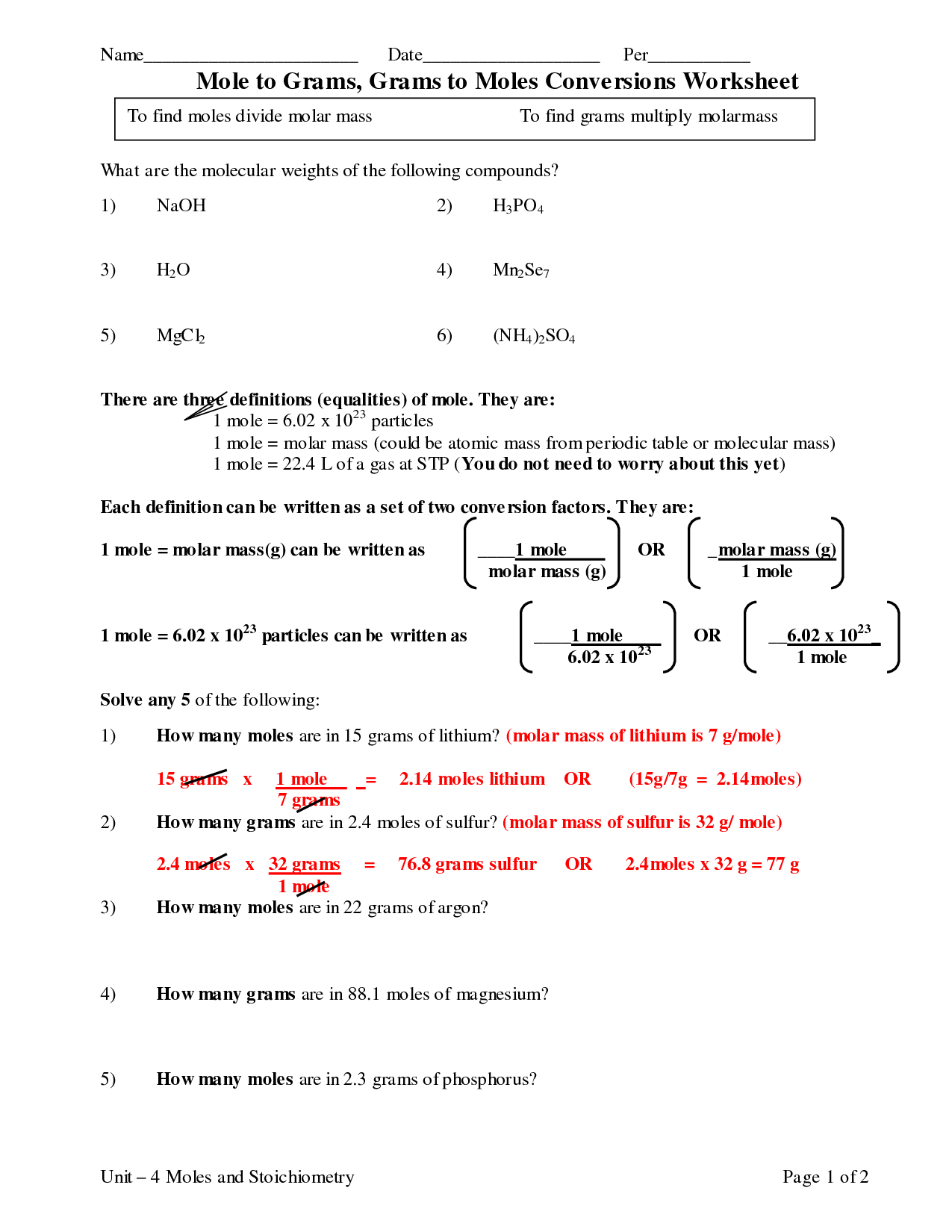
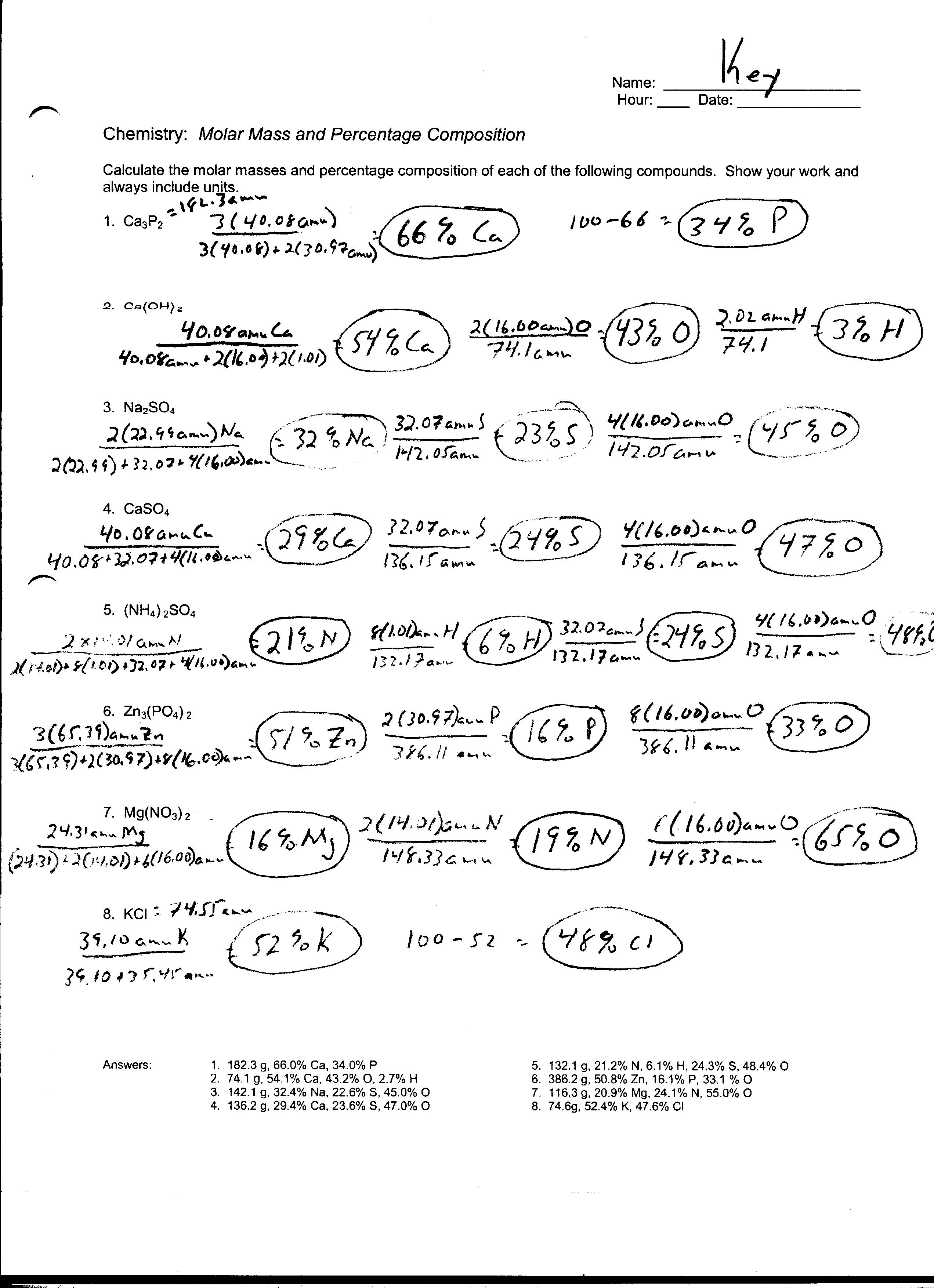
To calculate the number of moles of a substance when given the mass, you use the formula: moles = mass (in grams) / molar mass (in grams per mole). First, determine the molar mass of the substance by adding up the atomic masses of each element in the compound. Then, divide the given mass by the molar mass to find the number of moles. This calculation allows you to quantify the amount of substance present based on its mass in grams and its molar mass in grams per mole.
How do you convert moles to grams using molar mass?
To convert moles to grams using molar mass, you multiply the number of moles by the molar mass of the substance. The molar mass is the mass of one mole of a substance and is expressed in grams per mole. By multiplying the number of moles by the molar mass, you can determine the mass of the substance in grams. The formula for this conversion is: Mass (g) = Number of moles × Molar mass.
How can you convert moles to particles using Avogadro's number?
To convert moles to particles using Avogadro's number, you can multiply the number of moles by Avogadro's number. Avogadro's number is 6.022 x 10^23 particles/mol, so simply multiply the number of moles by 6.022 x 10^23 to get the number of particles. This conversion allows you to relate the amount of a substance in moles to the actual number of individual particles present.
What is the purpose of using mole-to-mole conversions in stoichiometry problems?
The purpose of using mole-to-mole conversions in stoichiometry problems is to determine the relationship between the reactants and products in a chemical reaction based on their balanced chemical equation. By converting the moles of one substance to moles of another, we can accurately calculate the amount of reactants needed, products formed, or other relevant quantities in a reaction to ensure complete understanding and efficient manipulation of chemical reactions.
How can you use mole-to-mole conversions to determine the limiting reactant in a chemical reaction?
To determine the limiting reactant in a chemical reaction using mole-to-mole conversions, first, balance the chemical equation. Then, convert the number of moles of each reactant given into moles of the desired product using the stoichiometry of the balanced equation. The reactant that produces the least amount of product, as calculated through mole-to-mole conversions, is the limiting reactant. This is because it is the reactant that is completely consumed first, limiting the amount of product that can be formed in the reaction.
Can mole-to-mole conversions be used to compare the amounts of different substances in a chemical reaction?
Yes, mole-to-mole conversions can be used to compare the amounts of different substances in a chemical reaction as they allow for the direct comparison of the number of molecules or ions of one substance to another based on their respective coefficients in the balanced chemical equation. This helps in determining the stoichiometry of the reaction and the amount of starting materials needed or products produced.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments