Mole Ratio 3 Page 10 Questions Worksheet Answers
Worksheets are an essential tool for students who are seeking a clear understanding of mole ratios and its application in chemistry. This Mole Ratio 3 page 10 questions worksheet provides a comprehensive set of exercises aimed at reinforcing the concept of mole ratios. By engaging with these carefully designed questions, students can effectively practice their skills and enhance their understanding of the subject.
Table of Images 👆
- Chapter 8 Covalent Bonding Worksheet Answer Key
- Stoichiometry Practice Worksheet Answer Key
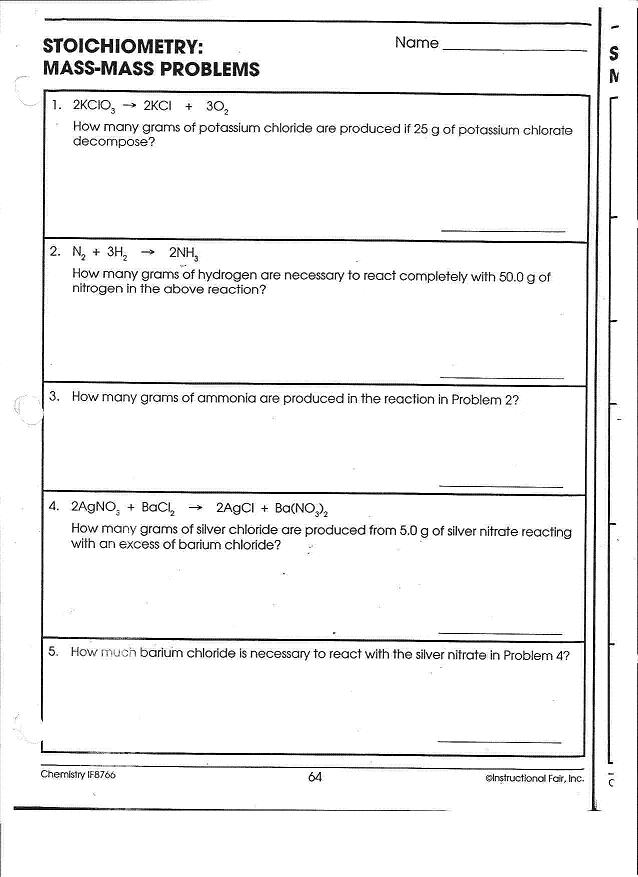
- Mole Ratio Worksheet Answer Key
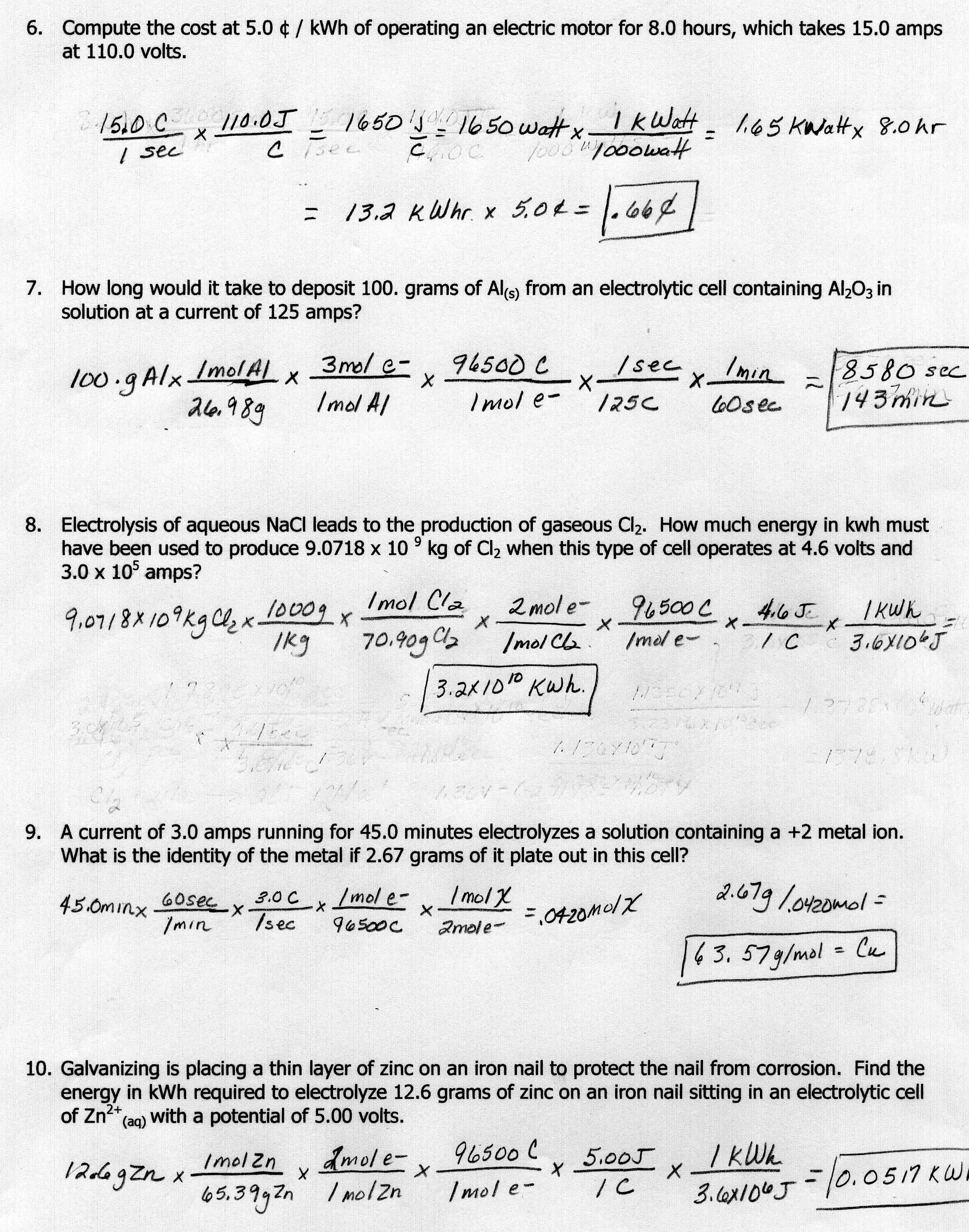
- Stoichiometry Worksheet 2 Answer Key
- Mole Conversion Worksheet Answers
- AP Chemistry Stoichiometry Worksheets
- Mole and the Relative Mass POGIL Answers
- Mass to Mole Calculations Worksheet
- Percent Composition and Molecular Formula Worksheet
- Stoichiometry Worksheet Answers
- Chemistry Worksheet Answer Keys
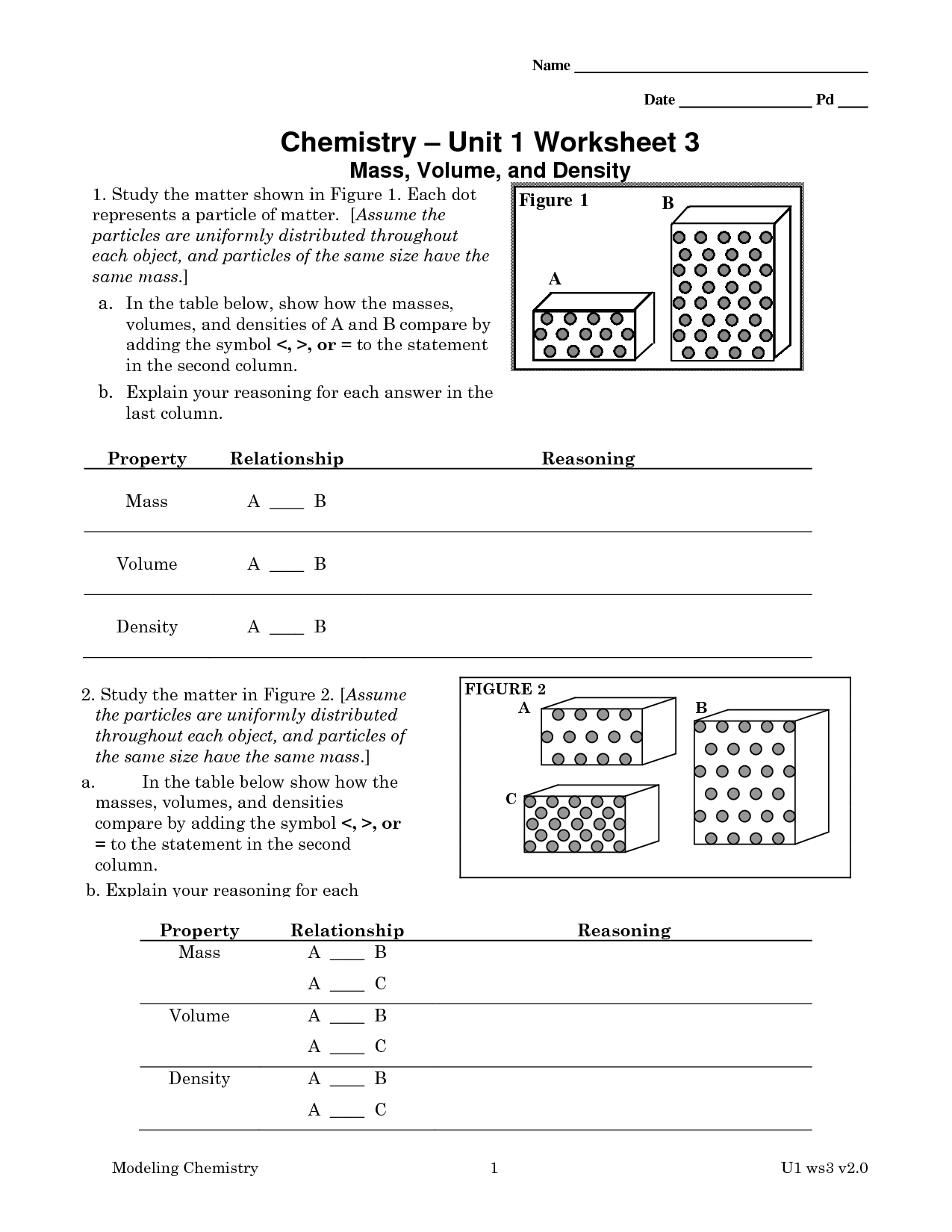
- Chemistry Unit 1 Worksheet 3
More Question Worksheets
Reading Labels Worksheets with QuestionsSimple Present Question Worksheet
100 Question Multiplication Worksheet
What is a mole ratio?
A mole ratio is the ratio of moles of one substance to moles of another substance in a balanced chemical equation. It represents the proportion of reactants and products in a chemical reaction and is used to calculate the quantities of substances involved in the reaction.
How is a mole ratio determined?
A mole ratio is determined by the coefficients of the balanced chemical equation. These coefficients represent the ratio of moles of each reactant and product involved in the reaction. By comparing the coefficients of the reactants and products in a balanced chemical equation, the mole ratio between different substances can be established, which is essential for stoichiometry calculations in chemistry.
Why are mole ratios important in stoichiometry?
Mole ratios are important in stoichiometry because they allow us to determine the relationship between the amounts of reactants and products in a chemical reaction. By using mole ratios, we can accurately calculate the amount of one substance needed to react completely with another, as well as predict the amount of product that will be formed. This helps in determining the most efficient way to carry out a reaction and ensures that the reaction proceeds in the desired manner.
How can mole ratios be used to calculate the amount of product formed in a chemical reaction?
Mole ratios can be used to calculate the amount of product formed in a chemical reaction by using the stoichiometry of the balanced chemical equation. By knowing the mole ratios between reactants and products, one can convert the moles of the reactant to the moles of the product using simple mathematics. This allows for the prediction of the amount of product that will be formed based on the amount of reactant that is consumed.
What is the relationship between mole ratios and balanced chemical equations?
Mole ratios in a balanced chemical equation indicate the proportions of reactants and products involved in the reaction. The coefficients in a balanced chemical equation represent these mole ratios, showing the exact amount of each substance involved in the reaction. By following these mole ratios, we can determine the quantities of substances consumed or produced in a reaction, helping us to calculate stoichiometry and predict the outcome of chemical reactions.
How can mole ratios be used to determine the limiting reactant in a reaction?
Mole ratios can be used to determine the limiting reactant in a reaction by comparing the actual ratio of moles of reactants present to the stoichiometric ratio of the reactants in the balanced chemical equation. The reactant that produces the smallest amount of product based on the mole ratio is the limiting reactant. By calculating the moles of each reactant involved in the reaction and comparing them using the mole ratios from the balanced equation, one can identify which reactant is present in the smallest amount and thus limits the amount of product that can be formed.
How is the concept of mole ratio applied in empirical formulas?
The concept of mole ratio is applied in determining empirical formulas by comparing the moles of each element present in a compound. This involves expressing the ratio of the moles of each element to the smallest whole number ratio, which then represents the subscripts in the empirical formula. By using the mole ratios, one can find the simplest whole number ratio of elements in a compound, allowing for the determination of the empirical formula based on the relative amounts of elements present.
Can mole ratios be used to convert between different units of measurement (e.g. grams to moles)?
Yes, mole ratios can be used to convert between different units of measurement such as grams to moles. By using the molar mass of a compound, which relates the mass of the compound to the number of moles present, one can set up conversion factors based on the mole ratio of the compound's components. This enables the conversion between grams and moles, allowing for easier calculations in chemical reactions and other related scenarios.
How do mole ratios help in determining the theoretical yield of a chemical reaction?
Mole ratios are used in stoichiometry to convert between the amounts of reactants and products involved in a chemical reaction. By knowing the mole ratios of the reactants and products, you can calculate the theoretical yield of a reaction, which is the maximum amount of product that can be formed based on the amounts of reactants used. Using mole ratios allows you to determine the precise amount of product that should be produced if the reaction goes to completion, helping you to predict and compare the actual yield of a reaction to its theoretical yield.
Is it possible to manipulate mole ratios to obtain a desired outcome in a reaction?
Yes, it is possible to manipulate mole ratios to obtain a desired outcome in a reaction by adjusting the amounts of reactants used in the reaction. By changing the mole ratios of reactants, you can shift the equilibrium towards the desired product, increase reaction yield, or control the extent of a reaction. This can be achieved by adding excess reactants, using stoichiometry to determine the necessary amounts of reactants, or manipulating reaction conditions such as temperature and pressure.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments