Lewis and Bohr Model Worksheet
Are you a science teacher or a student looking for a comprehensive and practical way to learn about Lewis and Bohr models? If so, this Lewis and Bohr Model Worksheet is just what you need. This worksheet is designed to help you understand the concepts of electron arrangements and chemical bonding through a series of engaging exercises and activities.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
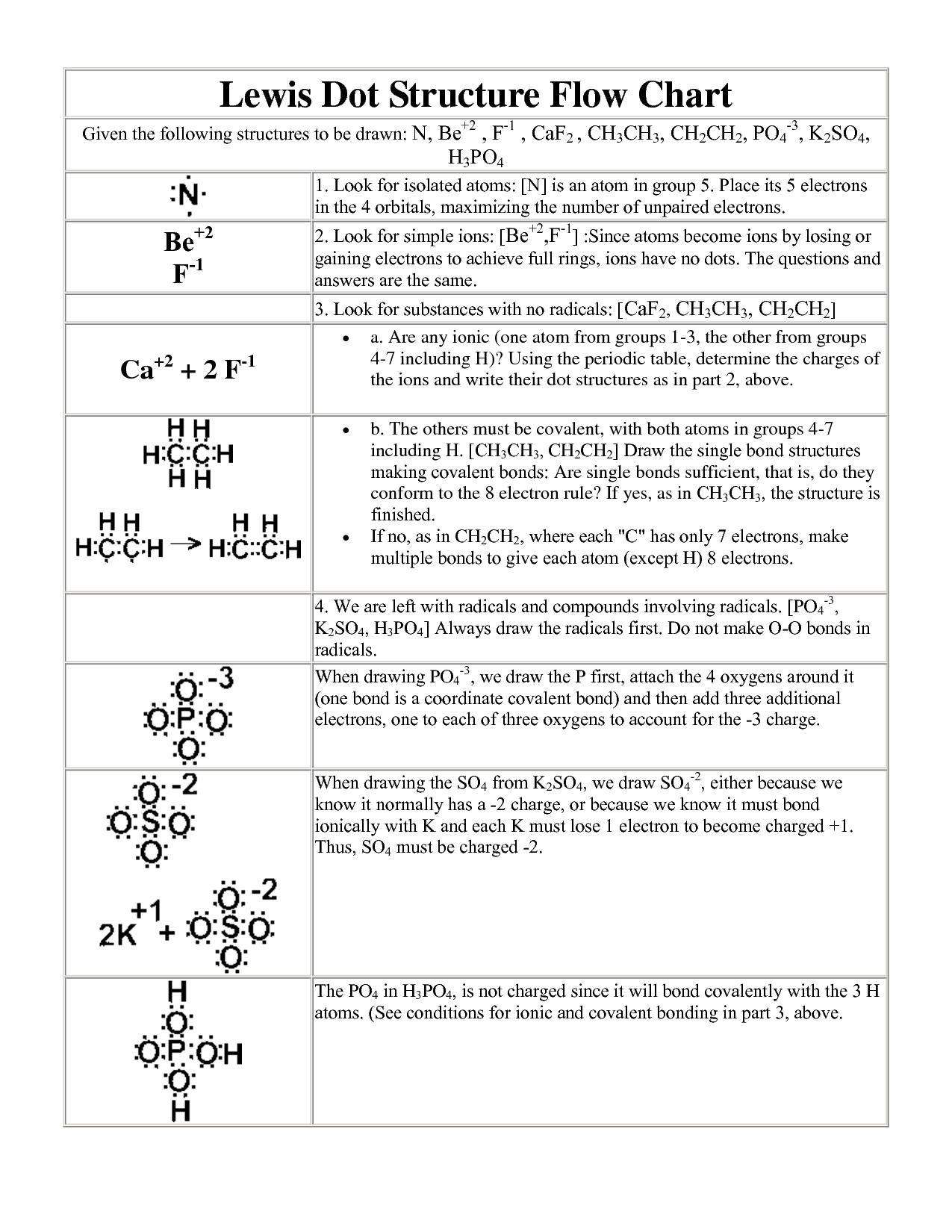
What is the Lewis model?
The Lewis model, or the Lewis dual-sector model, is an economic theory proposed by economist W. Arthur Lewis. The model describes a developing economy's transition from a traditional agricultural sector with low productivity and wages to a modern industrial sector with higher productivity and wages. It emphasizes the mobilization of surplus labor from the agricultural sector to the industrial sector as a key driver of economic development in developing countries.
What is the Bohr model?
The Bohr model is a model of the atom proposed by Danish physicist Niels Bohr in 1913. It suggests that electrons orbit the nucleus in specific energy levels or shells, similar to the way planets orbit the sun. These energy levels are quantized, meaning electrons can only occupy specific allowed energy levels and can jump between them by absorbing or emitting energy. This model helped to explain the stability of atoms and their spectral lines, laying the foundation for quantum mechanics.
What do Lewis structures show?
Lewis structures show the arrangement of atoms and valence electrons in a molecule or ion. They provide a visual representation of how the atoms are bonded in a molecule, showing the sharing or transfer of electrons between atoms to achieve a more stable electron configuration. Lewis structures help in understanding the connectivity of atoms, predicting molecular geometry, and determining the overall charge of the molecule.
How are Lewis structures helpful in understanding the chemical properties of elements?
Lewis structures are helpful in understanding the chemical properties of elements by providing a visual representation of the valence electrons and bonding patterns within a molecule. By examining a Lewis structure, one can determine the number of bonding and nonbonding electrons, as well as the types of bonds present (single, double, or triple). This information is crucial in predicting molecular geometry, polarity, reactivity, and overall chemical behavior of a compound. In essence, Lewis structures help chemists analyze and predict how elements will interact with one another based on their electronic configurations, which ultimately affects their chemical properties.
Describe the main concept behind the Bohr model.
The main concept behind the Bohr model is that electrons orbit the nucleus of an atom in specific energy levels or shells, rather than in random paths. These energy levels are quantized, meaning electrons can only exist in certain discrete energy levels. Electrons can jump from one energy level to another by absorbing or emitting specific amounts of energy in the form of photons. This model helped explain the stability of atoms and the spectral lines emitted by different elements.
What does the Bohr model explain about electron behavior in an atom?
The Bohr model explains that electrons in an atom orbit the nucleus in specific, quantized energy levels or orbits. Electrons can only exist in these allowed energy levels and can jump between them by absorbing or emitting energy in the form of photons. This model also accounts for the stability of atoms and explains why certain elements emit specific wavelengths of light when excited.
Explain the role of energy levels in the Bohr model.
In the Bohr model, electrons are arranged in specific energy levels around the nucleus of an atom. These energy levels represent the energy that an electron needs to maintain a stable orbit around the nucleus. The electrons can only exist in these quantized energy levels and move between them by absorbing or emitting specific amounts of energy in the form of photons. This concept helps to explain the stability of atoms and the emission and absorption of light in atomic systems, laying the foundation for the development of quantum mechanics.
How many energy levels are typically shown in the Bohr model?
In the Bohr model, there are typically a few discrete energy levels shown for an atom, which are represented by concentric circles around the nucleus. These energy levels are often labeled with integers starting from the innermost level as n=1, n=2, and so on, corresponding to different energy states available to the electrons in an atom.
What is the significance of the valence shell in the Lewis model?
The valence shell in the Lewis model is significant because it determines the number of electrons available for bonding with other atoms. The electrons in the valence shell are involved in forming chemical bonds, which ultimately determine the reactivity and chemical properties of an atom. The Lewis model simplifies the understanding of chemical bonding by focusing on the valence electrons, highlighting their role in the formation of stable structures through the sharing or transfer of electrons between atoms.
How do Lewis and Bohr models complement each other in understanding atomic structure and chemical reactivity?
The Lewis and Bohr models complement each other by providing different perspectives on atomic structure and chemical reactivity. The Lewis model focuses on the outer electron configuration of atoms, helping to predict bonding patterns and molecular shapes in covalent compounds. On the other hand, the Bohr model emphasizes the arrangement of electrons in energy levels around the nucleus, providing insights into the electronic structure and stability of atoms. By combining these two models, scientists can gain a more comprehensive understanding of atomic behavior, chemical bonding, and reactivity in a variety of chemical systems.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


































Comments