Ionic Compounds Worksheet Answers
Ionic compounds are an essential topic for any chemistry student to grasp, as they form the foundation of many chemical reactions. Whether you're a student struggling to understand the concept or a teacher looking for resources to support your lesson plans, having an Ionic Compounds Worksheet with accurate and detailed answers is crucial. This worksheet will provide you with the opportunity to solidify your understanding of the subject, test your knowledge, and reinforce key concepts.
Table of Images 👆
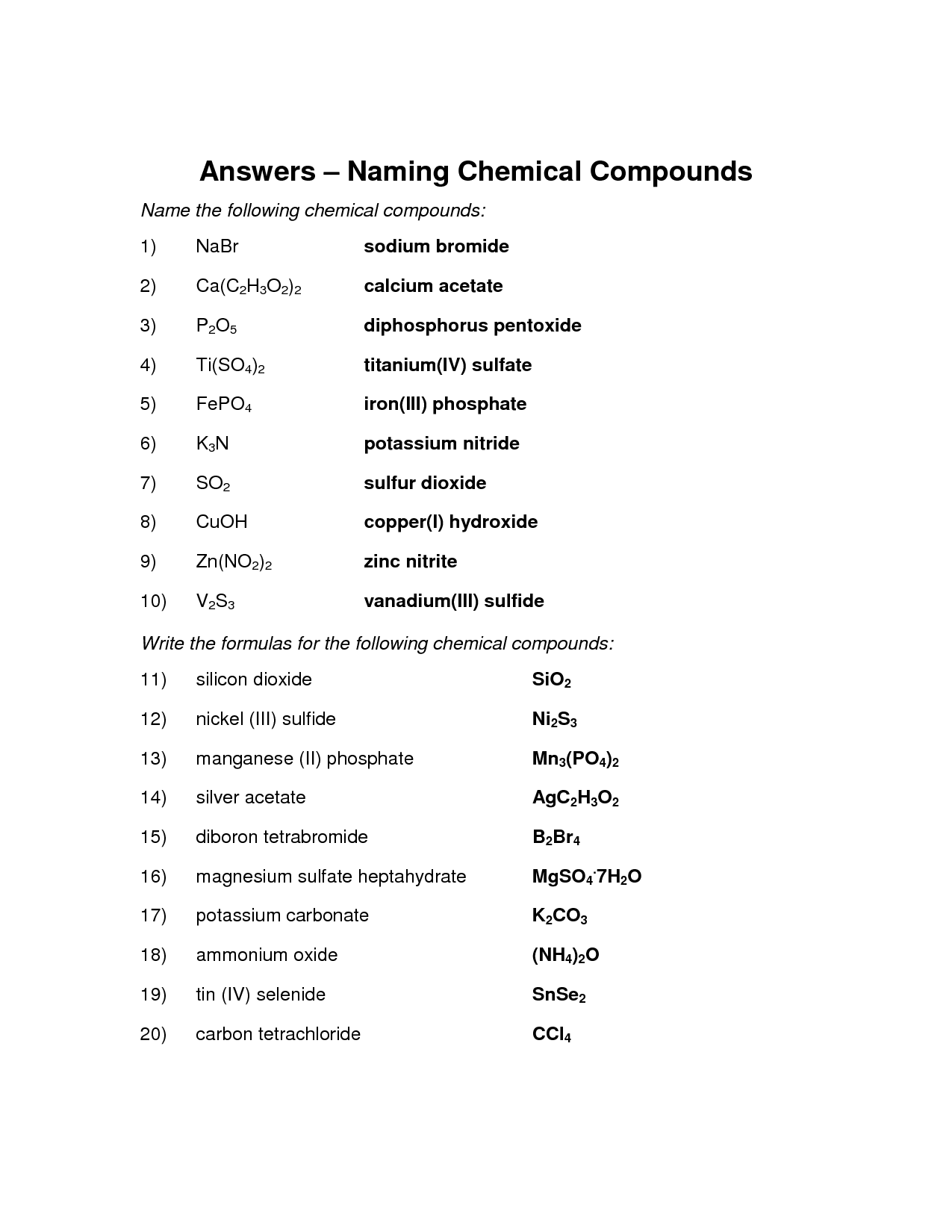
- Naming Chemical Compounds Worksheet Answers
- Practice Naming Ionic Compounds Worksheet Answers
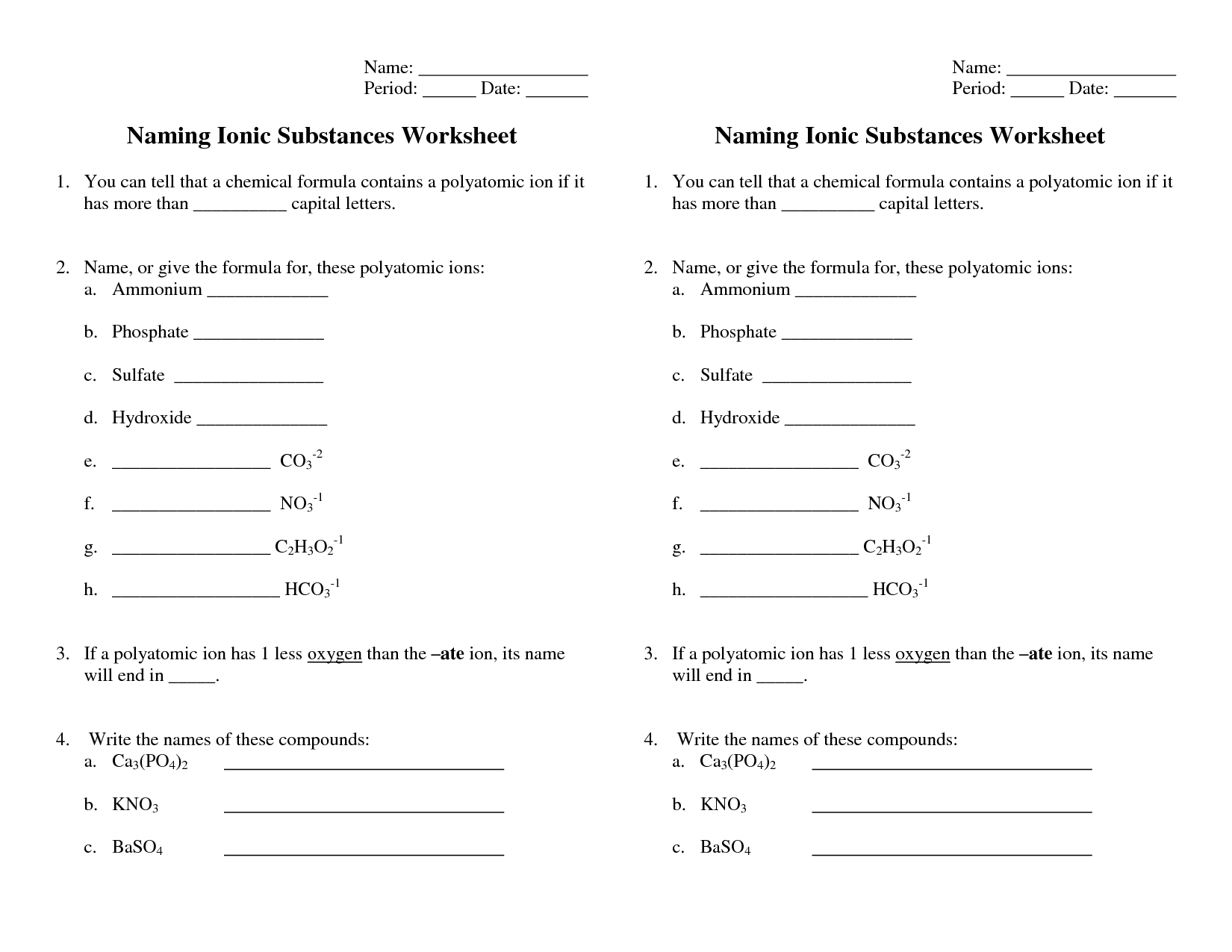
- Naming Ionic Compounds Worksheet Answers
- Net Ionic Equation Worksheet Answers
- Naming Ionic Compounds Worksheet Answer Key
- Writing Formulas for Binary Ionic Compounds Worksheets
- Polyatomic Ionic Compounds Worksheet
- Naming Compounds Worksheet
- Lewis Dot Structure Worksheet Answers
- 7th Grade Science Cells Worksheets
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What are ionic compounds?
Ionic compounds are chemical compounds composed of positively charged ions (cations) and negatively charged ions (anions) that are held together by electrostatic attraction. This strong attraction between oppositely charged ions allows them to form solid structures with distinctive physical and chemical properties.
How do ionic compounds form?
Ionic compounds form through the transfer of electrons from one atom to another. When a metal atom loses one or more electrons to a nonmetal atom, they become positively and negatively charged ions, respectively. These oppositely charged ions are then attracted to each other and form ionic bonds, creating a stable compound.
What is the difference between a cation and an anion?
A cation is a positively charged ion that is formed when an atom loses electrons, resulting in more protons than electrons. An anion, on the other hand, is a negatively charged ion that forms when an atom gains electrons, resulting in more electrons than protons. In summary, cations have a positive charge, while anions have a negative charge.
How are ionic compounds represented in chemical formulas?
Ionic compounds are represented in chemical formulas by using the symbols of the ions involved, with the cation (positively charged ion) listed first followed by the anion (negatively charged ion). The charges of each ion must balance out to ensure overall neutrality of the compound. The subscripts are used to indicate the ratio of ions present in the compound.
What is the significance of the charges on ions in ionic compounds?
The charges on ions in ionic compounds are significant because they determine how the ions interact and form stable compounds through the attraction of opposite charges. The charges on ions indicate the number of electrons gained or lost by an atom to achieve a full outer electron shell, resulting in a net positive or negative charge. This charge imbalance allows for the strong electrostatic forces of attraction between the positively and negatively charged ions, leading to the formation of crystal lattices in ionic compounds.
How do you determine the formula of an ionic compound?
To determine the formula of an ionic compound, you need to consider the charges of the ions involved. The formula should have a neutral charge, so you balance the positive and negative charges by using subscripts to show the ratio of ions present. For example, in sodium chloride (NaCl), sodium has a +1 charge and chlorine has a -1 charge, so they combine in a 1:1 ratio to form a neutral compound. This process applies to all ionic compounds where the charges of the ions involved help determine the formula.
What are some properties of ionic compounds?
Ionic compounds have high melting and boiling points, are typically soluble in water, and form crystals. They conduct electricity when dissolved in water or melted because they contain charged ions. Ionic compounds often have a crystal lattice structure and show brittleness. Additionally, they are usually formed between metals and nonmetals through the transfer of electrons.
How do ionic compounds dissolve in water?
Ionic compounds dissolve in water through a process called dissociation, where the polar water molecules surround the individual ions and pull them apart from each other due to their opposite charges. This results in the formation of aqueous solutions with the ions dispersed throughout the water, a process essential for ionic compounds to conduct electricity and participate in various chemical reactions.
How do ionic compounds conduct electricity?
Ionic compounds conduct electricity when they are in a molten state or dissolved in a solution because the ions are free to move and carry an electric current. In a solid state, ionic compounds do not conduct electricity since the ions are held in a fixed position and cannot move.
What are some examples of common ionic compounds?
Some common examples of ionic compounds include sodium chloride (table salt), magnesium oxide, potassium iodide, calcium carbonate (calcium in antacids), and aluminum sulfate (used in water purification).
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments