Ionic Bonding Worksheet Answer Key
Are you struggling to find a reliable answer key for your ionic bonding worksheet? Look no further! In this blog post, we will provide you with a comprehensive answer key that covers all the essential concepts and problems related to ionic bonding. Whether you are a student looking for additional practice or a teacher searching for a helpful resource to supplement your lesson plans, our answer key will provide you with the entity and subject you need to confidently navigate the world of ionic bonding.
Table of Images 👆
- Ionic and Covalent Bonding Worksheet
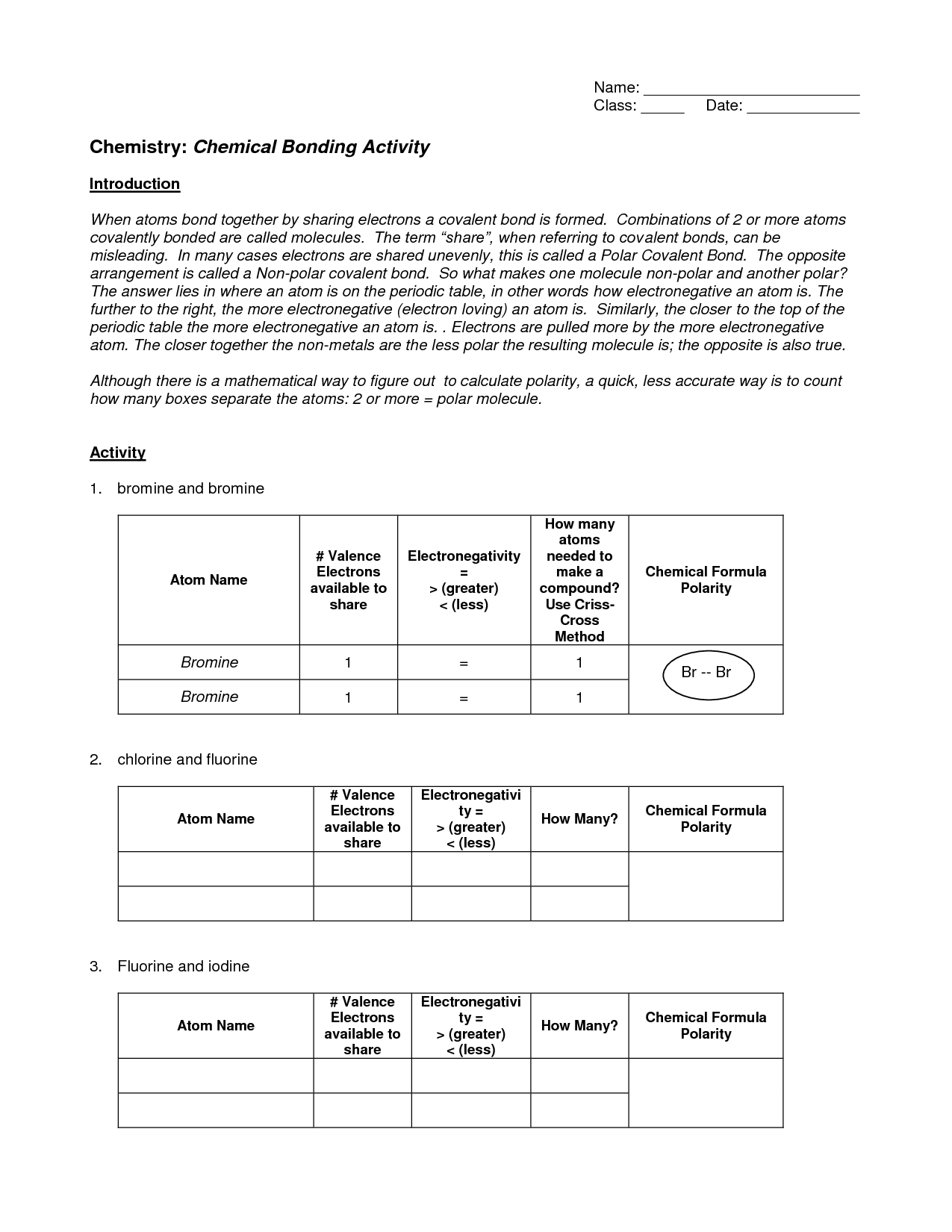
- Chemistry Chemical Bonding Worksheet Answers Activity
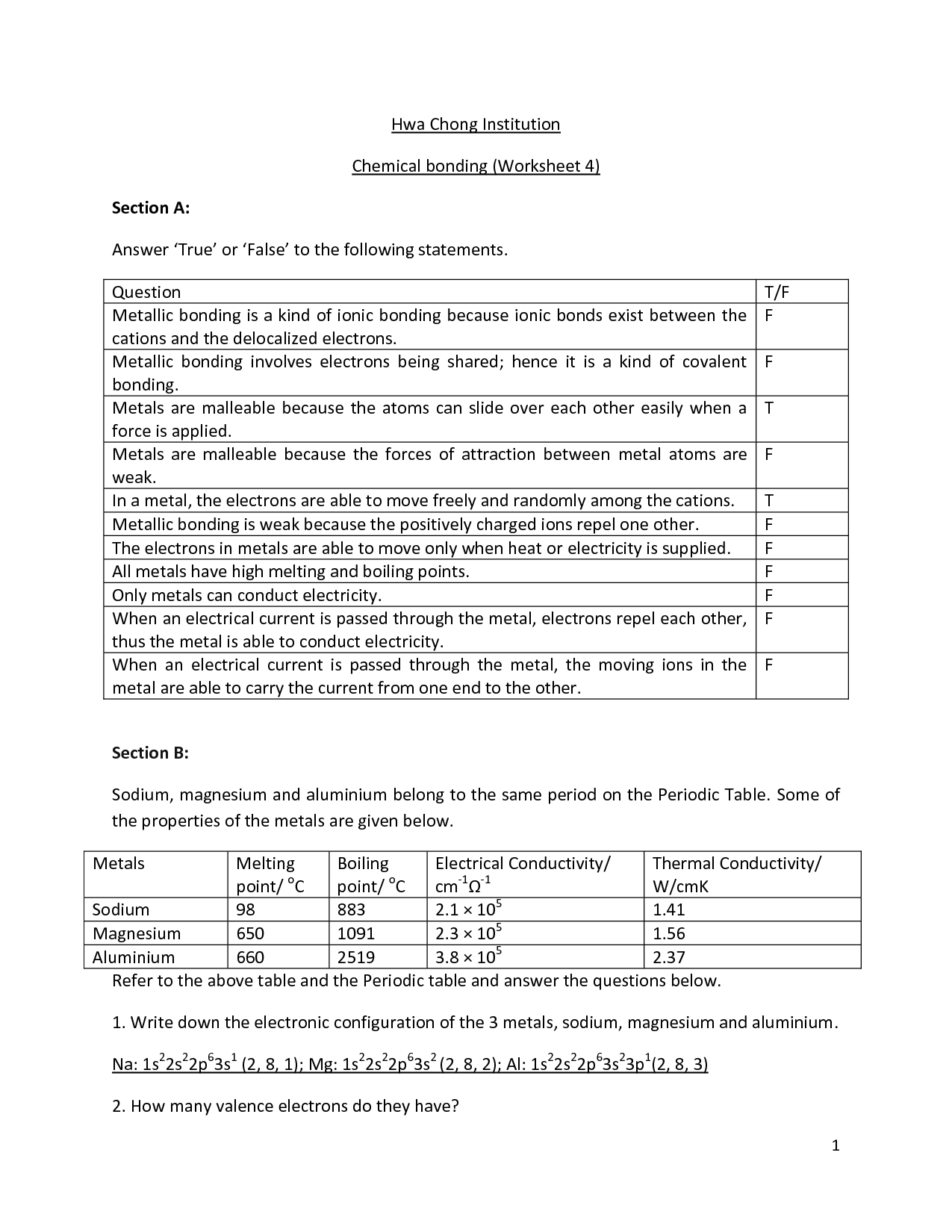
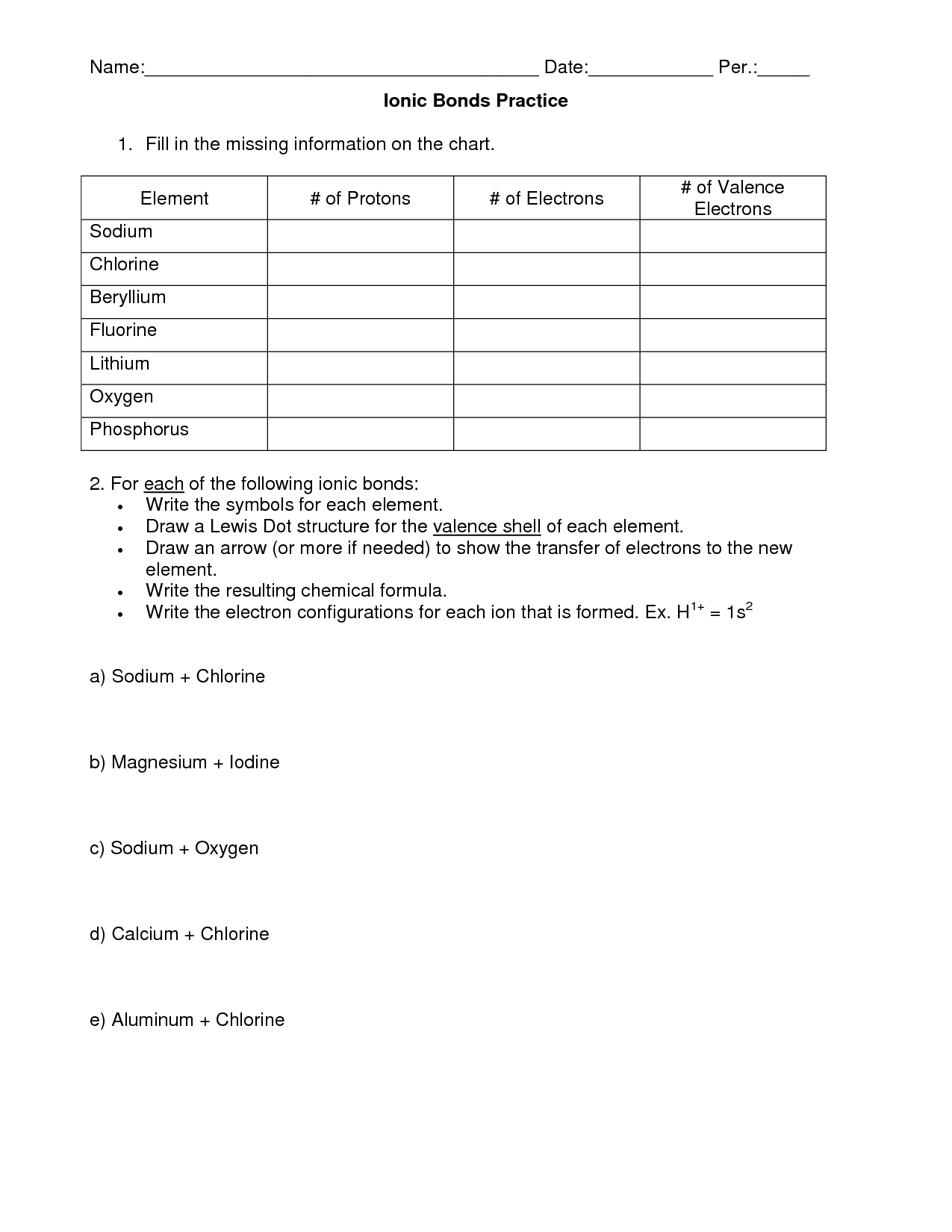
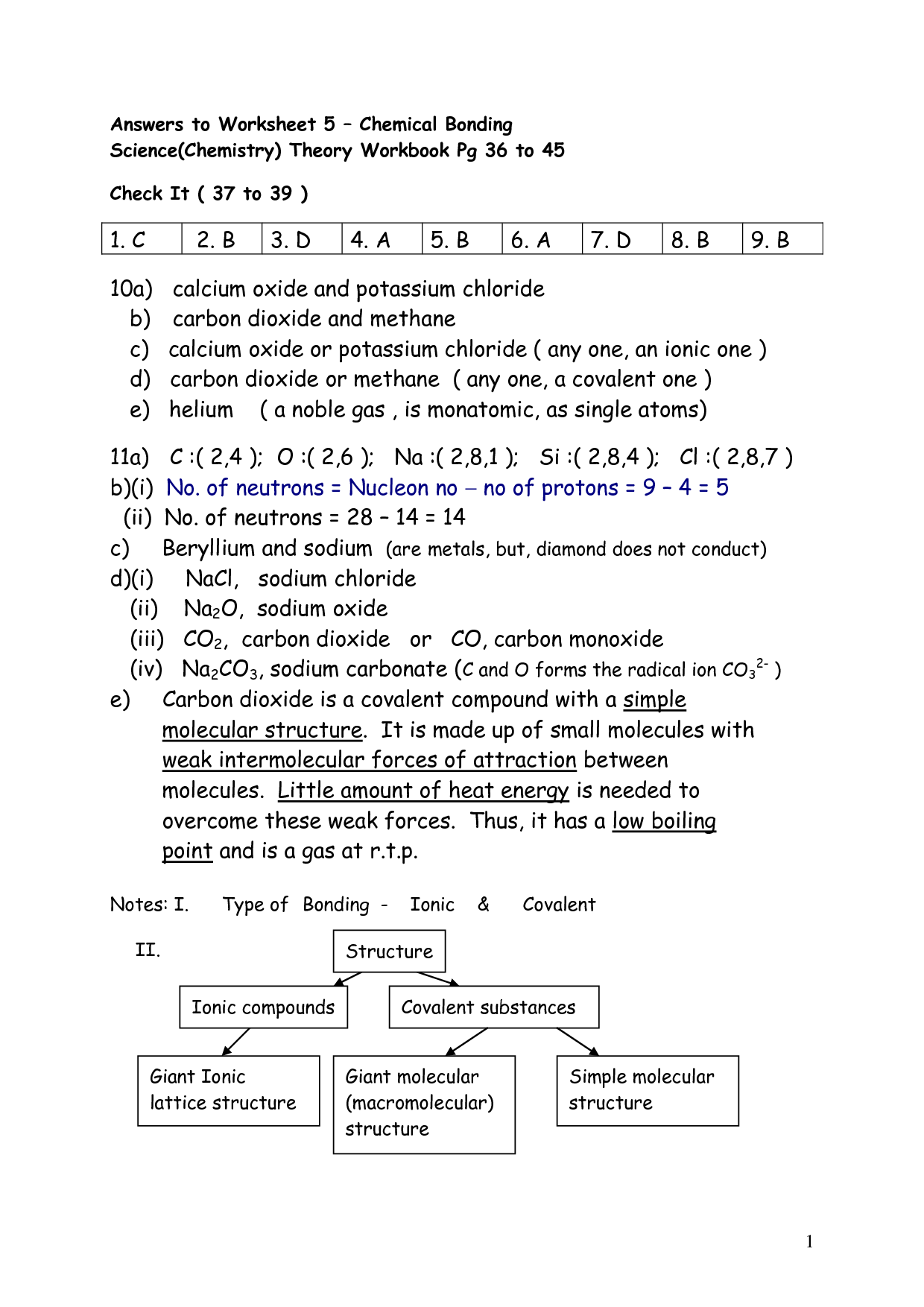
- Chemical Bonding Worksheet Answer Key
- Chemical Bonding Worksheet Answers
- ChemQuest 20 Ionic Bonding Worksheet Answer Key
- Student Exploration Ionic Bonds Gizmo Answer Key
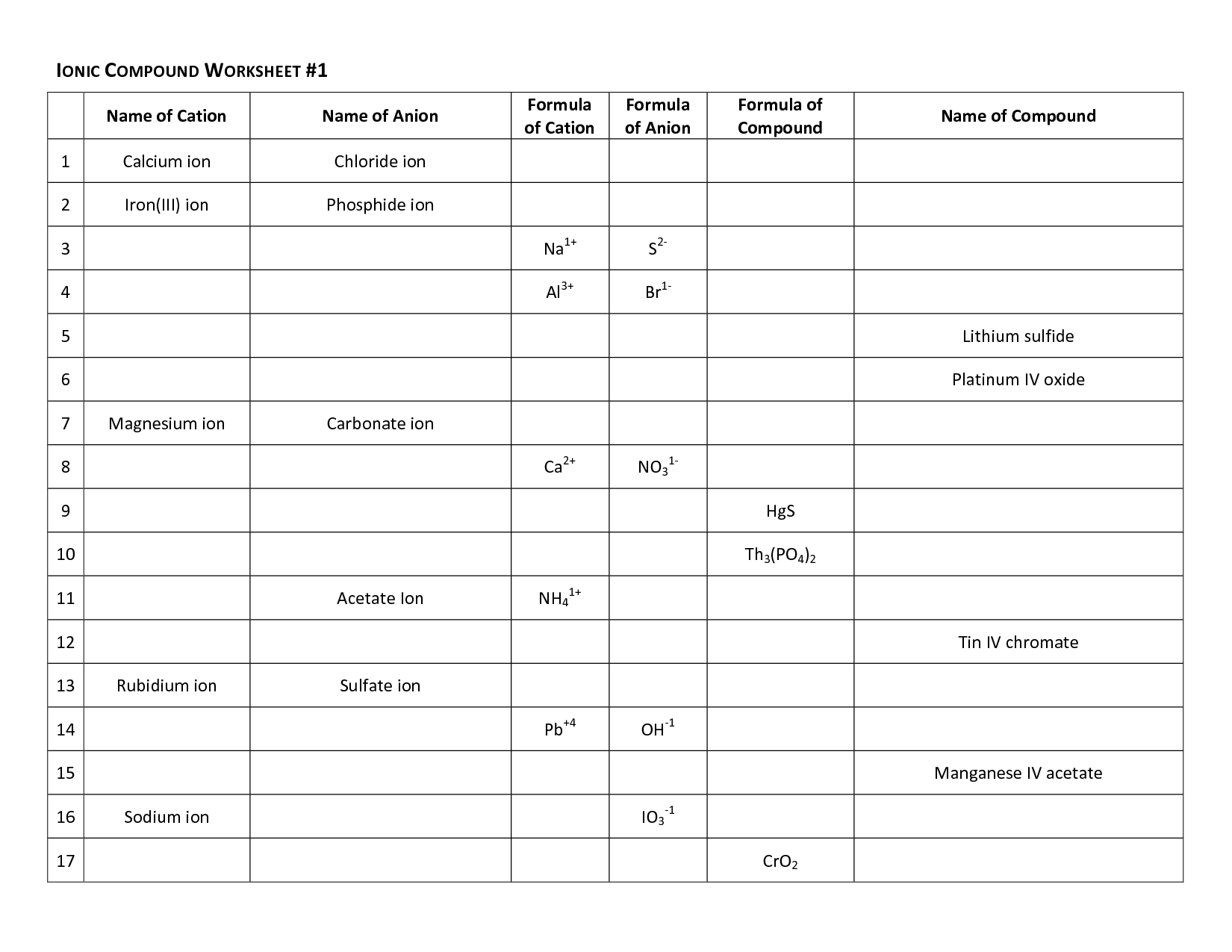
- Ionic Compound Worksheet 1 Answer Key
- Ionic and Covalent Bonds Worksheet
- Chapter 6 Chemical Bonding Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is an ionic bond?
An ionic bond is a type of chemical bond that forms when one atom transfers electrons to another atom, resulting in the formation of positive and negative ions. These ions are held together by electrostatic forces of attraction, creating a strong bond between the atoms. This bond typically occurs between a metal and a nonmetal, with the metal atom losing electrons to the nonmetal atom, leading to the creation of a positively charged cation and a negatively charged anion.
How do ionic bonds form?
Ionic bonds form when one atom transfers one or more electrons to another atom. This transfer creates positively charged ions (cations) and negatively charged ions (anions) that are attracted to each other due to their opposite charges, resulting in the formation of an ionic bond.
What are the two types of ions involved in an ionic bond?
The two types of ions involved in an ionic bond are cations, which are positively charged ions formed by losing electrons, and anions, which are negatively charged ions formed by gaining electrons. Cations are attracted to anions due to their opposite charges, leading to the formation of an ionic bond.
How is an ionic compound different from a covalent compound?
An ionic compound is formed through the electrical attraction between positively and negatively charged ions, while a covalent compound is formed through the sharing of electrons between atoms. Ionic compounds have high melting and boiling points, are usually soluble in water, and conduct electricity when dissolved or melted, whereas covalent compounds typically have lower melting and boiling points, are often insoluble in water, and do not conduct electricity.
What is an anion and how is it formed?
An anion is a negatively charged ion that is formed when an atom gains one or more electrons. Atoms typically gain electrons to achieve a stable electron configuration, usually by filling their outermost energy level. This results in the atom having a net negative charge, turning it into an anion.
What is a cation and how is it formed?
A cation is a positively charged ion formed when an atom loses one or more electrons. This loss of electrons creates an imbalance between the positively charged protons in the nucleus and the negatively charged electrons, resulting in a net positive charge. Cations are typically formed through processes such as ionization, where an atom loses an electron to become positively charged, or through chemical reactions that result in the transfer of electrons between atoms.
How are the charges of ions determined?
The charges of ions are determined by the number of electrons they have gained or lost compared to the number of protons in their nucleus. If an ion has gained electrons, it will have a negative charge, while if it has lost electrons, it will have a positive charge. The magnitude of the charge is equal to the difference in the number of electrons and protons.
How do you determine the formula of an ionic compound?
To determine the formula of an ionic compound, you need to balance the charges of the ions involved. This involves knowing the charges of the cations and anions present in the compound, and then combining them in such a way that the overall charge is neutral. This is often done through the crisscross method, where the absolute value of the charge on one ion becomes the subscript for the other and vice versa. The final formula reflects the ratio in which the ions combine to achieve electrical neutrality.
What are some properties of compounds with ionic bonds?
Compounds with ionic bonds typically have high melting and boiling points, are soluble in water, conduct electricity in molten or aqueous state, and have crystal lattice structures. They also have strong electrostatic attractions between positively and negatively charged ions, leading to their characteristic properties of brittleness and easily shattering when subjected to force.
Can you provide an example of a compound with an ionic bond and describe its formation?
One example of a compound with an ionic bond is sodium chloride (NaCl), also known as table salt. Sodium chloride is formed through the transfer of electrons from a sodium atom (Na) to a chlorine atom (Cl). Sodium, which has one electron in its outer shell, readily donates this electron to chlorine, which has seven electrons in its outer shell and needs one more to achieve stability. This transfer of electrons results in the formation of Na+ cation and Cl- anion, which are attracted to each other through electrostatic forces, forming the ionic bond.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments