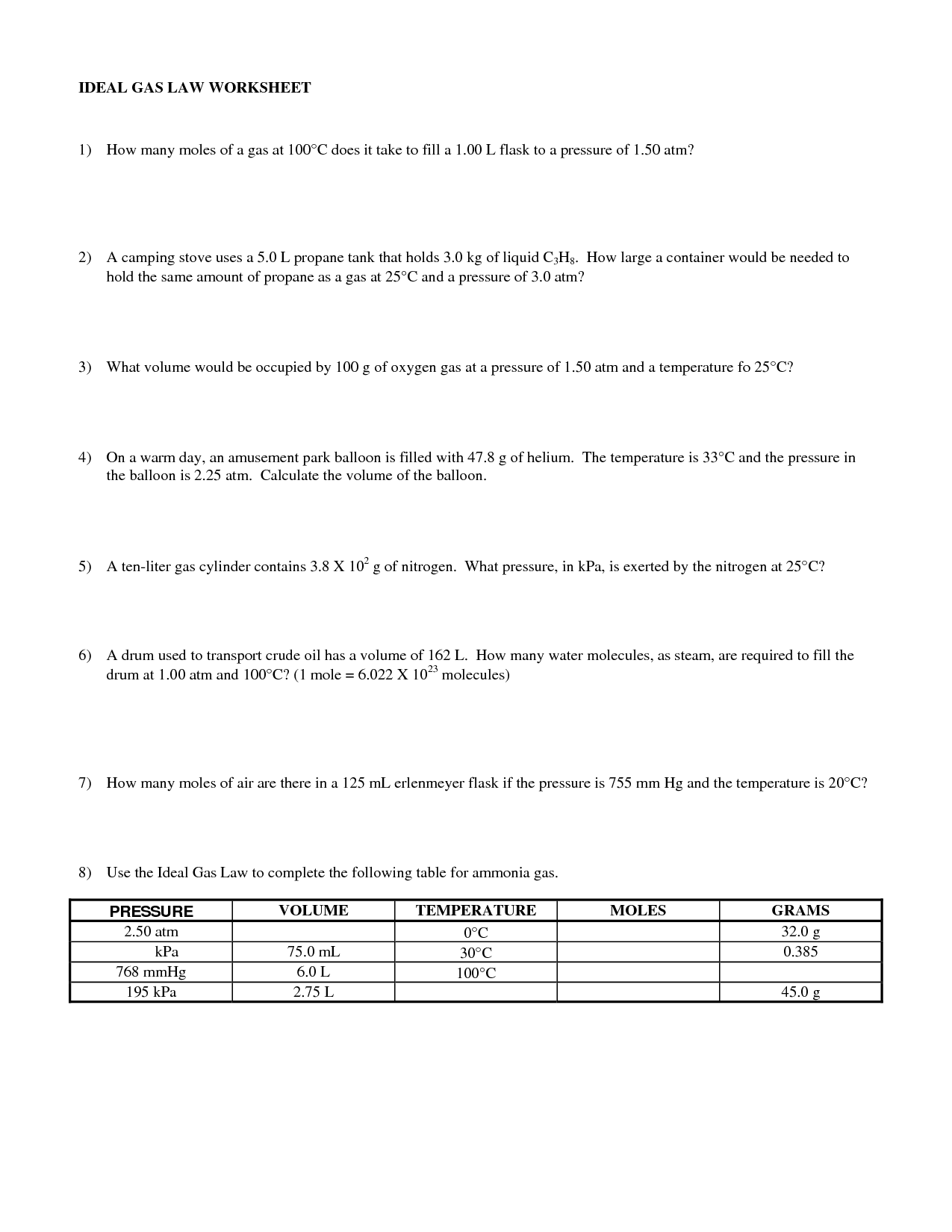
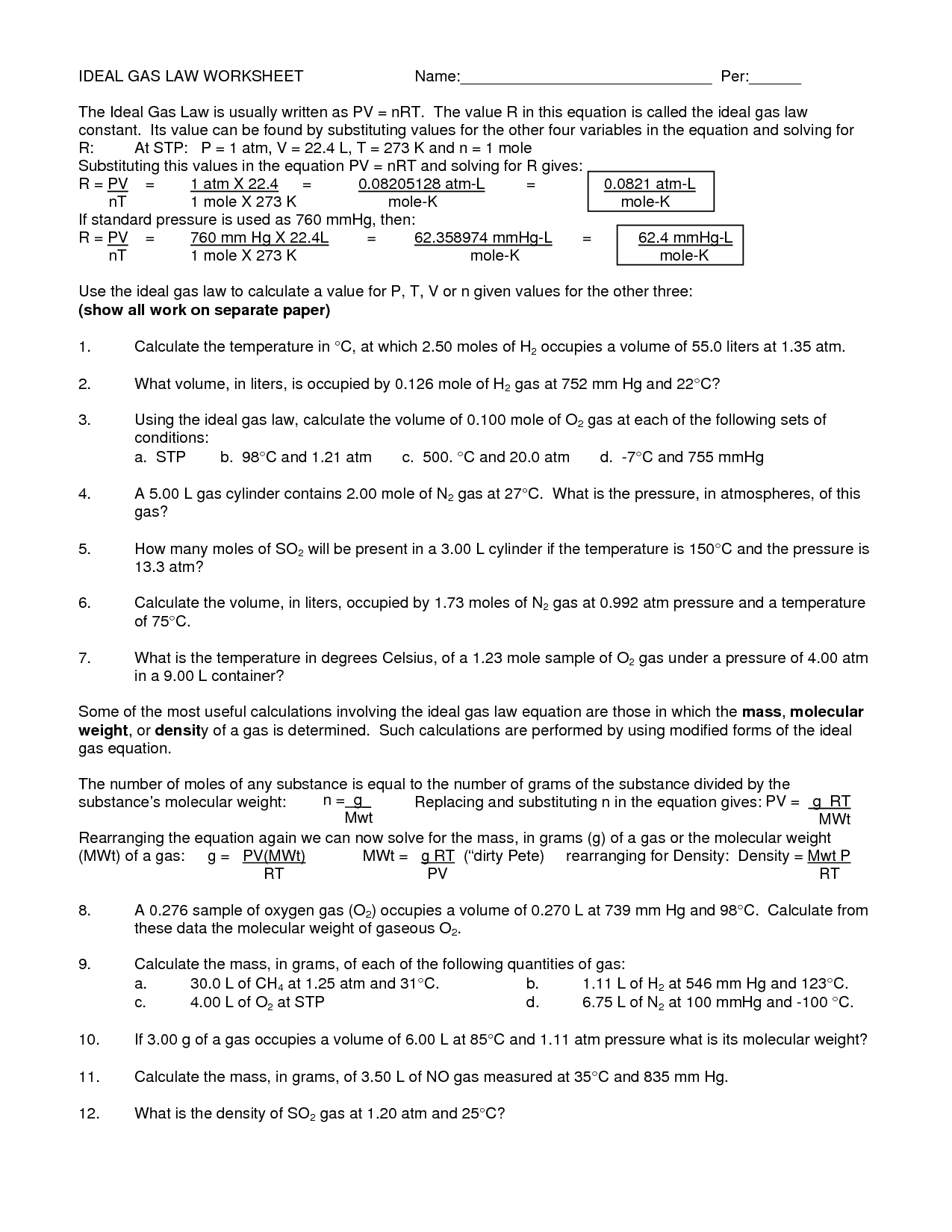
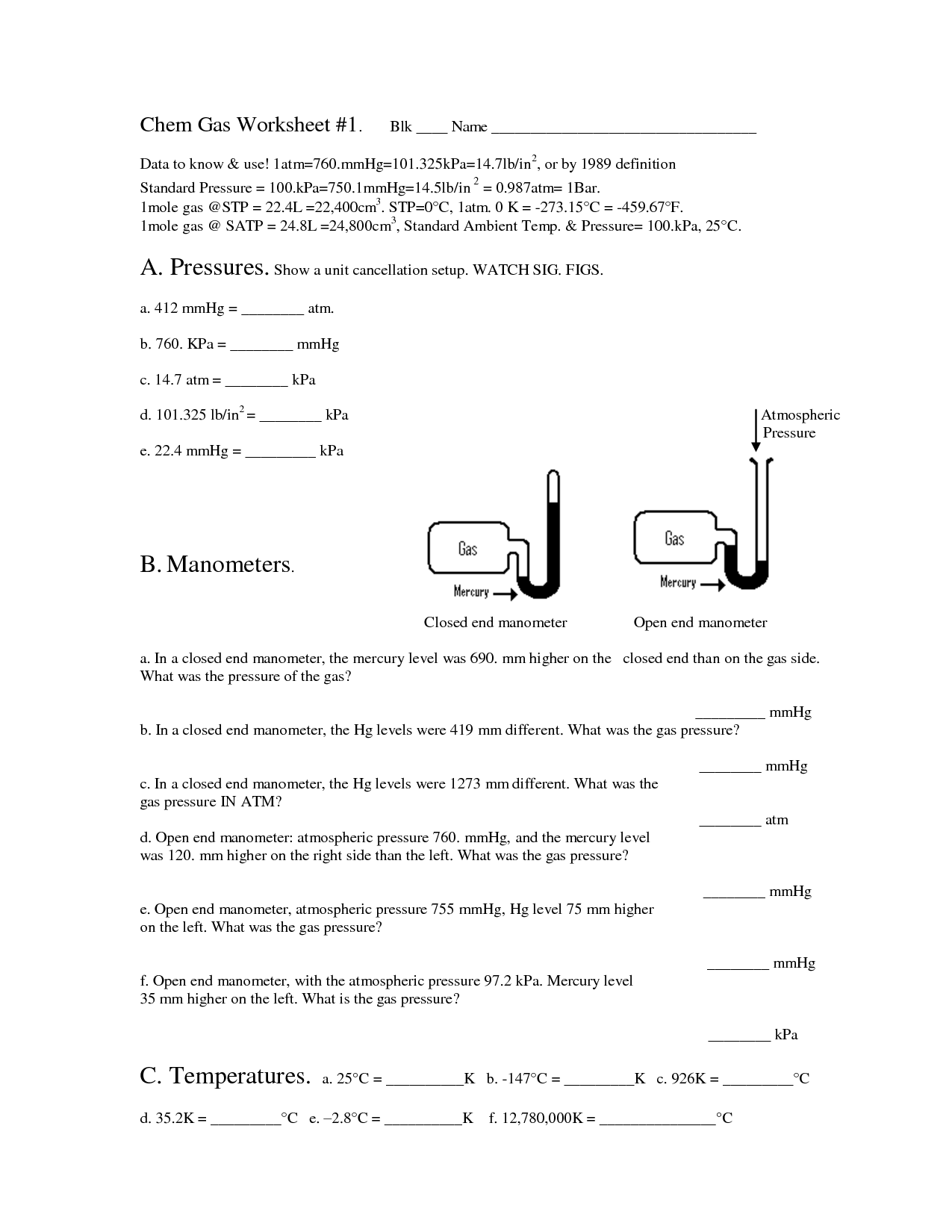
Ideal Gas Law Worksheet
The Ideal Gas Law worksheet serves as a valuable tool for students studying thermodynamics and gas laws. Designed to provide practice and enhance understanding, this worksheet explores the relationship between pressure, volume, temperature, and the number of moles of gas using the Ideal Gas Law equation. Whether you are a high school student preparing for an exam or a college student delving deeper into the subject, this worksheet brings clarity to the complexities of the Ideal Gas Law.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the ideal gas law?
The ideal gas law is a fundamental equation in thermodynamics that describes the relationship between the pressure, volume, and temperature of an ideal gas. It is expressed as PV = nRT, where P is the pressure of the gas, V is the volume it occupies, n is the number of moles of the gas, R is the ideal gas constant, and T is the absolute temperature.
What does each letter in the ideal gas law equation represent?
In the ideal gas law equation, PV = nRT, P represents pressure, V represents volume, n represents the number of moles of gas, R represents the gas constant, and T represents temperature.
How does temperature affect the pressure of an ideal gas?
According to the ideal gas law, pressure is directly proportional to temperature for a fixed amount of gas at constant volume. This means that as temperature increases, the average kinetic energy of gas molecules increases, leading to more frequent and forceful collisions with the walls of the container, resulting in an increase in pressure. Conversely, as temperature decreases, the average kinetic energy and collisions decrease, leading to a decrease in pressure.
How does volume affect the pressure of an ideal gas?
According to the ideal gas law, pressure is inversely proportional to volume when the temperature and the amount of gas are kept constant. This means that as volume increases, pressure decreases, and vice versa. This relationship is described by Boyle's Law, which states that for a given amount of gas at a constant temperature, the pressure and volume are inversely related.
How does the amount of gas (moles) affect the pressure of an ideal gas?
According to the ideal gas law, the pressure of an ideal gas is directly proportional to the number of gas moles present at a constant temperature and volume. This means that as the number of gas moles increases, the pressure of the gas will also increase, assuming the temperature and volume remain constant. Conversely, if the number of gas moles decreases, the pressure will decrease as well.
How does temperature affect the volume of an ideal gas?
According to the ideal gas law, an increase in temperature will cause the volume of an ideal gas to increase, assuming pressure and amount of gas remain constant. This is because an increase in temperature leads to an increase in the average kinetic energy of gas particles, causing them to move with greater speed and impact the container walls more frequently, resulting in an increase in volume. Conversely, a decrease in temperature would cause the volume of an ideal gas to decrease.
How does pressure affect the volume of an ideal gas?
According to Boyle's Law, pressure and volume are inversely related for an ideal gas at constant temperature. This means that as pressure increases, the volume of the gas decreases, and vice versa. So, when pressure is applied to an ideal gas, its volume will decrease proportionally, assuming the temperature remains constant.
How does the amount of gas (moles) affect the volume of an ideal gas?
According to Avogadro's law, the volume of an ideal gas is directly proportional to the amount of gas (moles) when pressure and temperature are kept constant. This means that as the amount of gas increases, the volume of the gas will also increase proportionally. Conversely, if the amount of gas decreases, the volume of the gas will decrease accordingly.
How does temperature affect the amount of gas (moles) of an ideal gas?
According to the ideal gas law, when the temperature of an ideal gas increases, the average kinetic energy of the gas particles also increases, causing the gas molecules to move faster and collide more frequently with the container walls. This results in an increase in the pressure exerted by the gas and therefore an increase in the number of gas moles present in the container, assuming volume and pressure are held constant. Conversely, when the temperature decreases, the average kinetic energy decreases, leading to a decrease in the pressure of the gas and a decrease in the amount of gas moles present in the container, again assuming volume and pressure are held constant.
How does pressure affect the amount of gas (moles) of an ideal gas?
According to Boyle's Law, at a constant temperature, the amount of gas (moles) of an ideal gas is inversely proportional to the pressure it is subjected to. This means that as the pressure on a gas increases, the volume of the gas decreases, leading to an increase in the gas molecules' effective collisions, causing the gas to be more compressed. Conversely, if the pressure decreases, the volume increases, which results in lower gas compression and the gas molecules having more space to move.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
































Comments