Ideal Gas Law Worksheet Answer Key
The Ideal Gas Law Worksheet Answer Key is a valuable resource that provides both entity and subject information for students studying the principles of gas behavior. With clear and concise explanations, this answer key serves as a tool to help students understand and apply the concepts of the Ideal Gas Law in a variety of problem-solving scenarios. Whether you are a high school chemistry student or a college-level science enthusiast, this worksheet answer key provides the perfect guide to enhance your understanding of gas laws.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
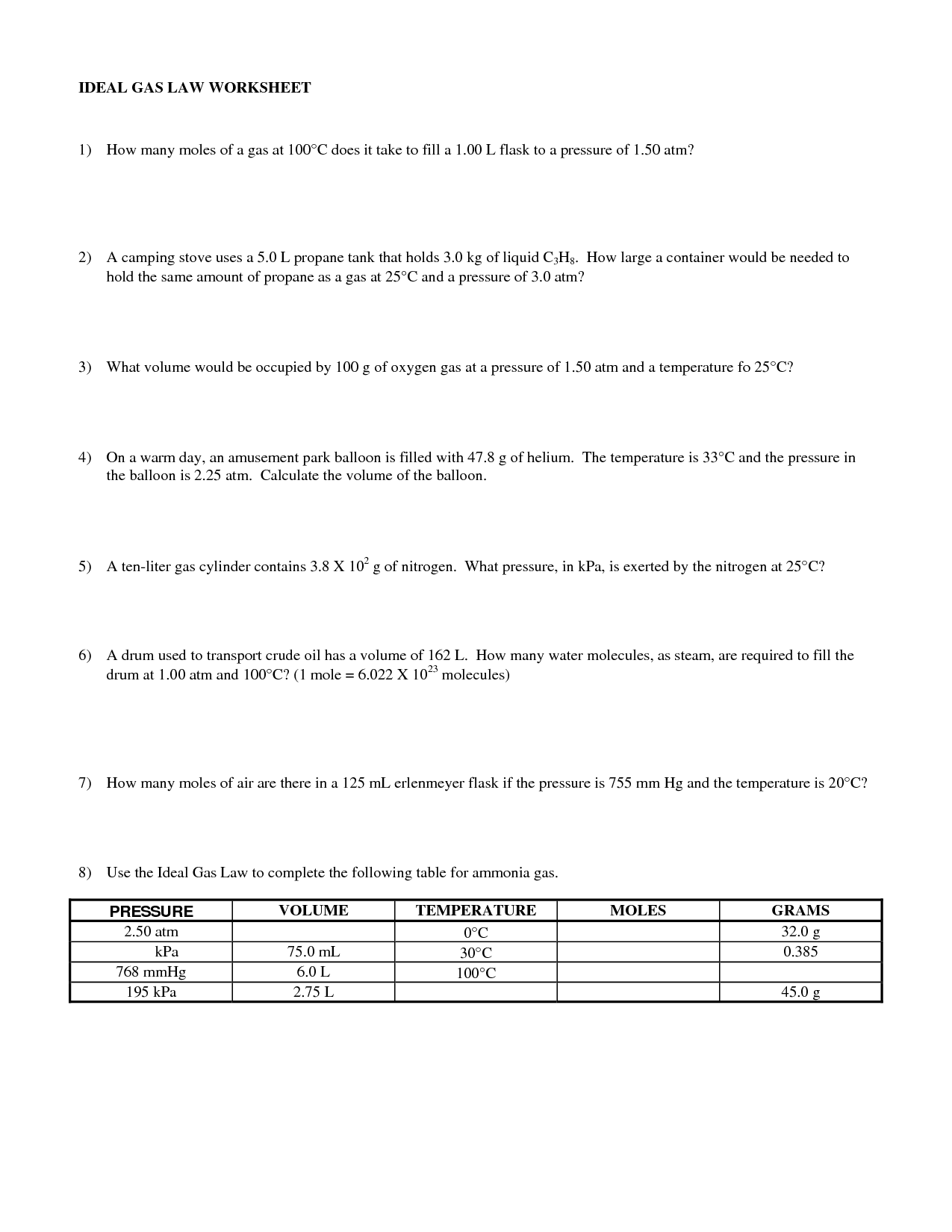
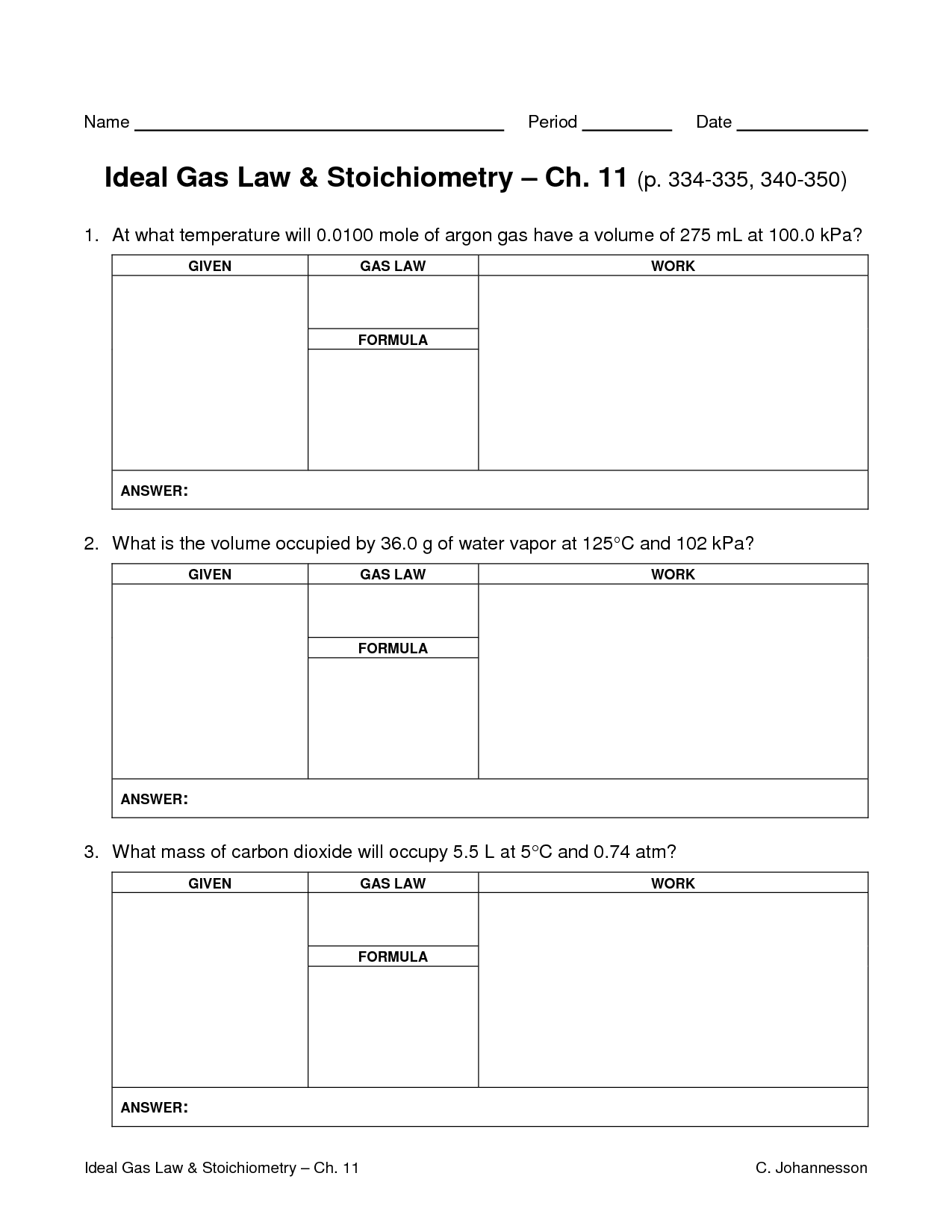
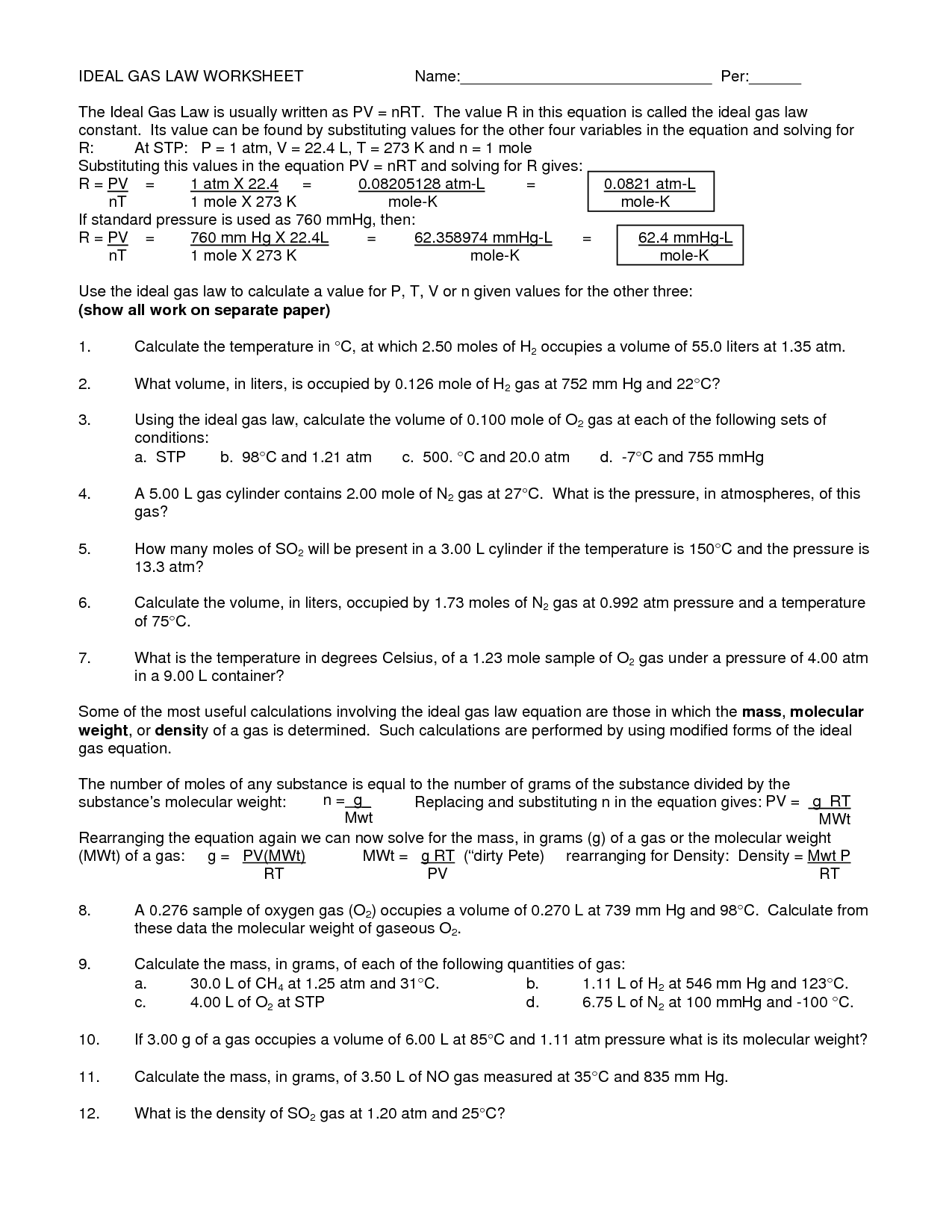
What is the ideal gas law?
The ideal gas law is a fundamental equation in thermodynamics that describes the relationship between the pressure, volume, and temperature of an ideal gas. It is represented by the equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in kelvin.
What are the variables in the ideal gas law equation?
The variables in the ideal gas law equation, PV = nRT, are pressure (P), volume (V), moles of gas (n), temperature (T), and the gas constant (R).
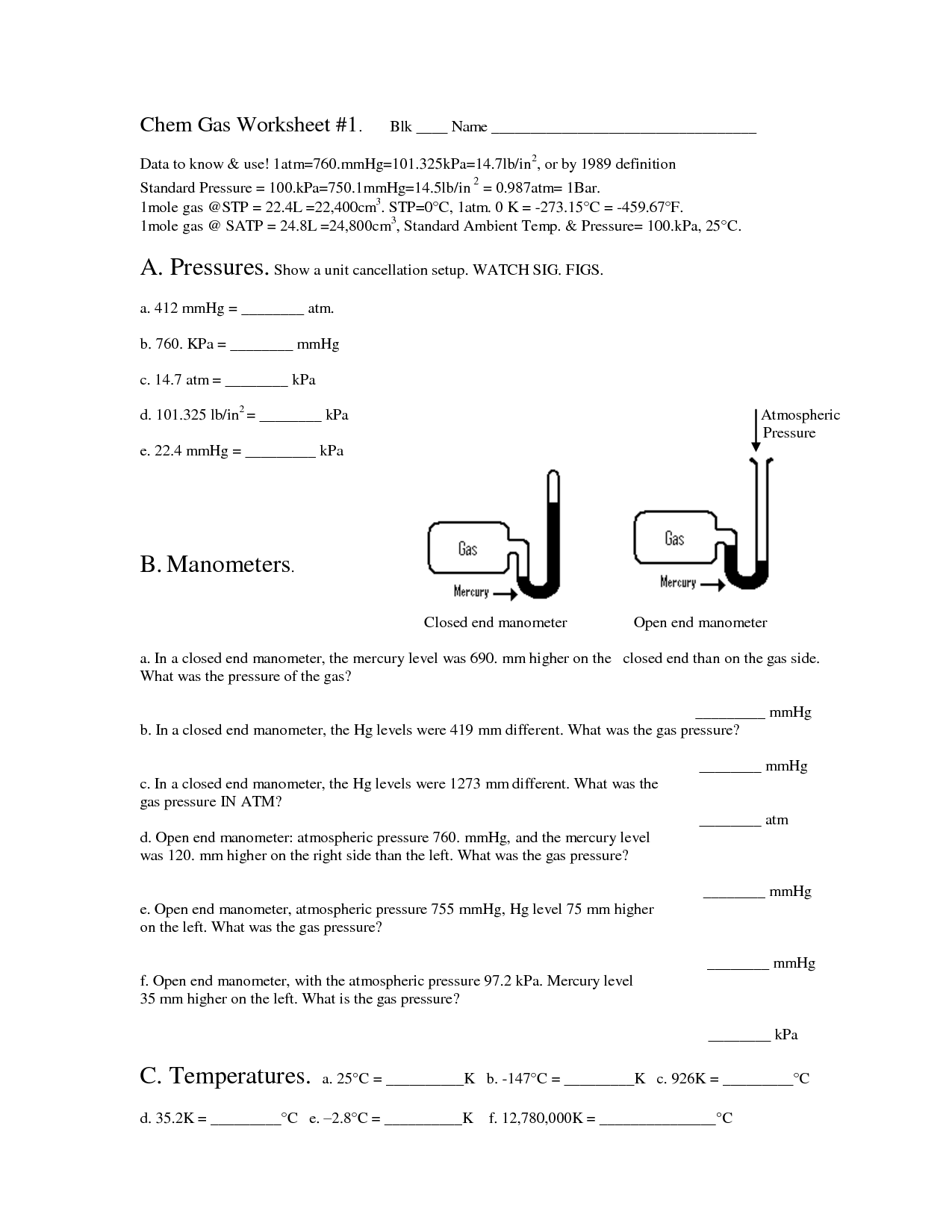
How is temperature measured in the ideal gas law?
In the ideal gas law, temperature is measured in Kelvin (K). This is because temperature must be in absolute terms when using the ideal gas law equation to ensure accurate calculations.
What is the significance of the ideal gas constant (R)?
The ideal gas constant (R) is a crucial constant in the ideal gas law equation, PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, T is the temperature in Kelvin, and R is the ideal gas constant. R helps relate the macroscopic properties of gases, such as pressure, volume, and temperature, to the microscopic behaviors of gas molecules. It enables us to calculate the behavior of gases under various conditions and has a wide range of applications in thermodynamics, chemistry, and physics.
How does the ideal gas law relate to Boyle's Law?
The ideal gas law, which relates the pressure, volume, and temperature of a gas, can be derived from Boyle's Law, along with Charles's Law and Avogadro's Law. Boyle's Law specifically states that at a constant temperature, the pressure and volume of a gas are inversely proportional, meaning that as one increases, the other decreases. This relationship is a fundamental component of the ideal gas law, as it demonstrates how changes in volume can affect pressure when temperature is held constant.
How does the ideal gas law relate to Charles's Law?
The ideal gas law, which states that the pressure of a gas is directly proportional to its temperature when the volume and amount of gas are constant, is related to Charles's Law because Charles's Law specifically focuses on the relationship between the volume and temperature of a gas while keeping pressure and amount of gas constant. Charles's Law states that the volume of a gas is directly proportional to its absolute temperature, meaning that as the temperature of a gas increases, its volume also increases proportionally. Thus, both laws describe how different gas properties are interconnected and can be used to predict and analyze the behavior of gases under various conditions.
How does the ideal gas law relate to Avogadro's Law?
The ideal gas law is a combination of several gas laws, including Avogadro's Law. Avogadro's Law states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. This relationship is reflected in the ideal gas law equation, which includes the constant "n" representing the number of moles of gas. Therefore, the ideal gas law incorporates Avogadro's Law by specifying the relationship between the volume of a gas and the number of molecules it contains under specific conditions of temperature and pressure.
What is the purpose of the ideal gas law in solving gas law problems?
The purpose of the ideal gas law in solving gas law problems is to relate the pressure, volume, amount of substance, and temperature of a gas to each other in a single equation. By using the ideal gas law, which states that PV = nRT, where P is pressure, V is volume, n is the amount of substance, R is the gas constant, and T is temperature, we can calculate missing variables and predict how changes in conditions will affect a gas system. This allows us to understand and make predictions about the behavior of gases under different conditions.
What are the units commonly used in the ideal gas law equation?
The units commonly used in the ideal gas law equation are pressure, typically in atmospheres (atm) or pascals (Pa); volume, typically in liters (L) or cubic meters (m^3); temperature, typically in kelvin (K); and the gas constant, which can be in various units depending on the specific value used (for example, 0.08206 L·atm/(K·mol) or 8.314 J/(mol·K)).
How does the ideal gas law account for the behavior of real gases?
The ideal gas law accounts for the behavior of real gases by assuming ideal conditions of low pressure and high temperature where gas molecules have no volume and do not interact with each other. In reality, real gases have volume and experience intermolecular forces, leading to deviations from ideal behavior. Correction factors, such as the compressibility factor or van der Waals equation, are used to account for the differences between ideal and real gas behavior by considering factors such as molecular size, attractive forces, and pressure.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments