Heat and Specific Heat Capacity Worksheet
Are you struggling to understand the concept of heat and specific heat capacity? Look no further, as this worksheet will provide you with a comprehensive understanding of these topics. Whether you are a student studying physics or someone curious about the properties of heat, this worksheet will guide you through the fundamental principles, definitions, and equations related to heat and specific heat capacity.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is heat?
Heat is a form of energy that is transferred between objects due to a temperature difference. It is typically transferred by conduction, convection, or radiation and is responsible for increasing the temperature of an object or changing its state of matter.
How is heat different from temperature?
Heat and temperature are related but different concepts. Temperature is a measure of the average kinetic energy of the particles in a substance, indicating how hot or cold it is. Heat, on the other hand, is the energy transfer that occurs between substances due to a temperature difference. In simpler terms, temperature is a measure of the internal energy of a system, while heat is the transfer of that energy from one system to another.
What is specific heat capacity?
Specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one degree Celsius (or one Kelvin). It is a physical property that differs between different materials and helps determine how effectively a substance can store and release heat energy.
How is specific heat capacity related to the amount of heat required to raise the temperature of an object?
Specific heat capacity is a measure of the amount of heat energy required to raise the temperature of a substance by a certain amount. It represents the ability of a material to absorb heat without significantly changing its temperature. The higher the specific heat capacity of a material, the more heat energy is required to increase its temperature. This means that substances with high specific heat capacities require more heat to raise their temperature compared to those with lower specific heat capacities.
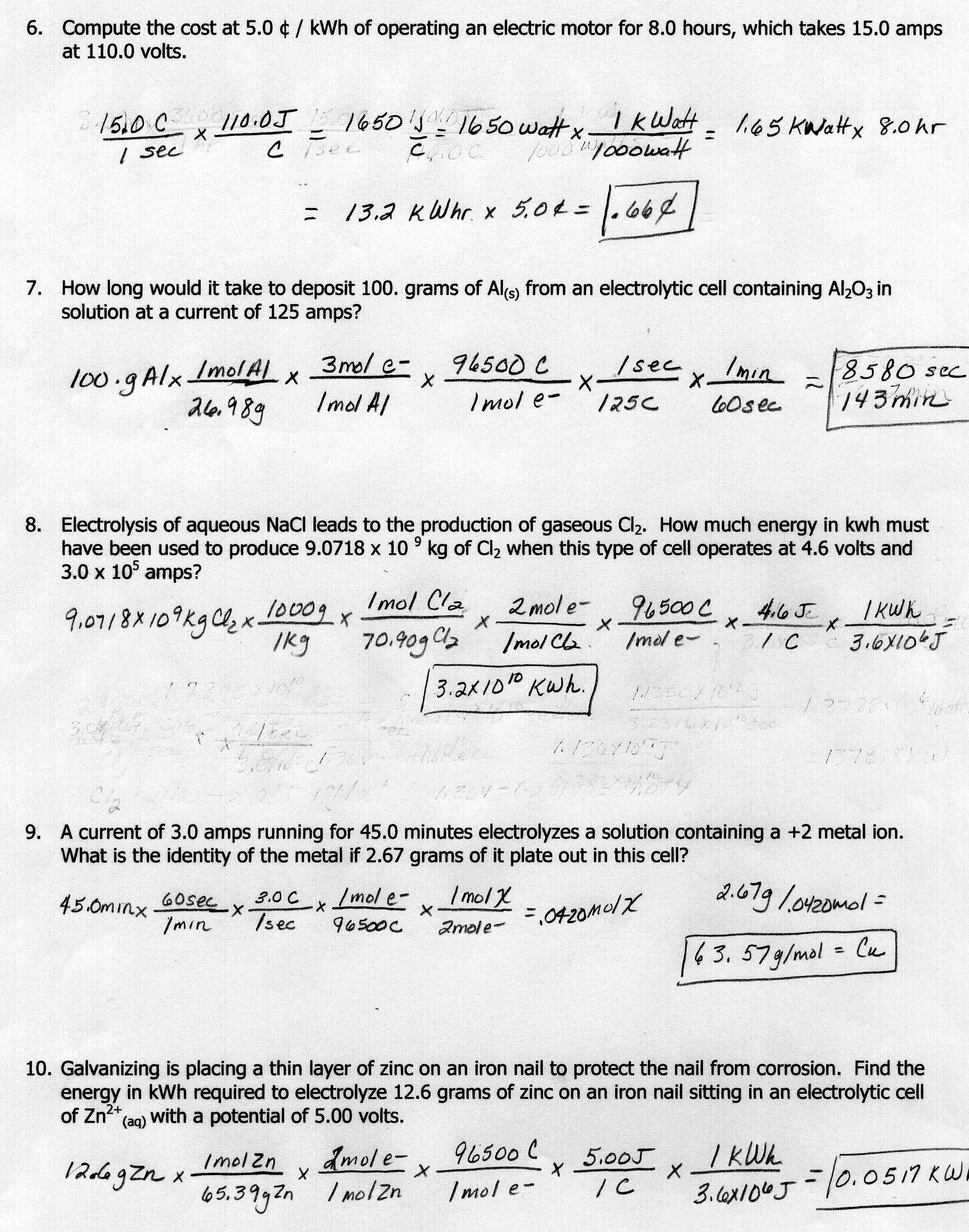
What is the equation used to calculate heat energy?
The equation used to calculate heat energy is Q = mc?T, where Q represents the heat energy, m is the mass of the object, c is the specific heat capacity of the substance, and ?T is the change in temperature of the object.
What are some common units of measurement for heat and specific heat capacity?
Some common units of measurement for heat are calories, joules, BTUs (British Thermal Units), and electron volts. For specific heat capacity, the common units are joules per gram-degree Celsius (J/g°C) or joules per kilogram-degree Celsius (J/kg°C), as well as calories per gram-degree Celsius (cal/g°C) or calories per kilogram-degree Celsius (cal/kg°C).
How does specific heat capacity differ between different substances?
Specific heat capacity is a measure of the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius. Different substances have different specific heat capacities because they have different molecular structures and compositions. For example, substances with complex molecular structures, such as water, tend to have higher specific heat capacities compared to simpler substances like metals. This is because complex structures require more energy to raise their temperature due to the need to overcome stronger intermolecular forces. Additionally, substances with higher specific heat capacities generally have a greater ability to absorb and retain heat energy, making them more effective at regulating temperature changes in their surroundings.
How does specific heat capacity affect the transfer of heat between objects?
Specific heat capacity measures the amount of heat energy required to raise the temperature of a material by a certain amount. Objects with higher specific heat capacities require more heat energy to increase their temperature compared to objects with lower specific heat capacities. This means that materials with higher specific heat capacities transfer heat more slowly between objects, as they absorb and hold onto heat energy for a longer period before temperature change occurs. Conversely, materials with lower specific heat capacities transfer heat more quickly as they heat up and cool down faster.
How does specific heat capacity impact the effectiveness of different materials as insulators?
Specific heat capacity impacts the effectiveness of different materials as insulators by influencing their ability to store and release heat. Materials with high specific heat capacity can absorb and retain more heat energy, making them better insulators as they require more energy to increase their temperature. Conversely, materials with low specific heat capacity heat up and cool down quickly, leading to poor insulation properties. Therefore, materials with high specific heat capacity are more effective insulators as they can better regulate temperature changes and reduce heat transfer.
How is the concept of specific heat capacity used in practical applications, such as heating and cooling systems?
The concept of specific heat capacity is used in practical applications like heating and cooling systems to determine how much heat energy is required to raise or lower the temperature of a substance. By knowing the specific heat capacity of a material, engineers can accurately calculate the amount of energy needed to heat or cool a space efficiently. This information is crucial for designing heating and cooling systems that are energy-efficient and cost-effective, ensuring optimal performance while minimizing waste.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

































Comments