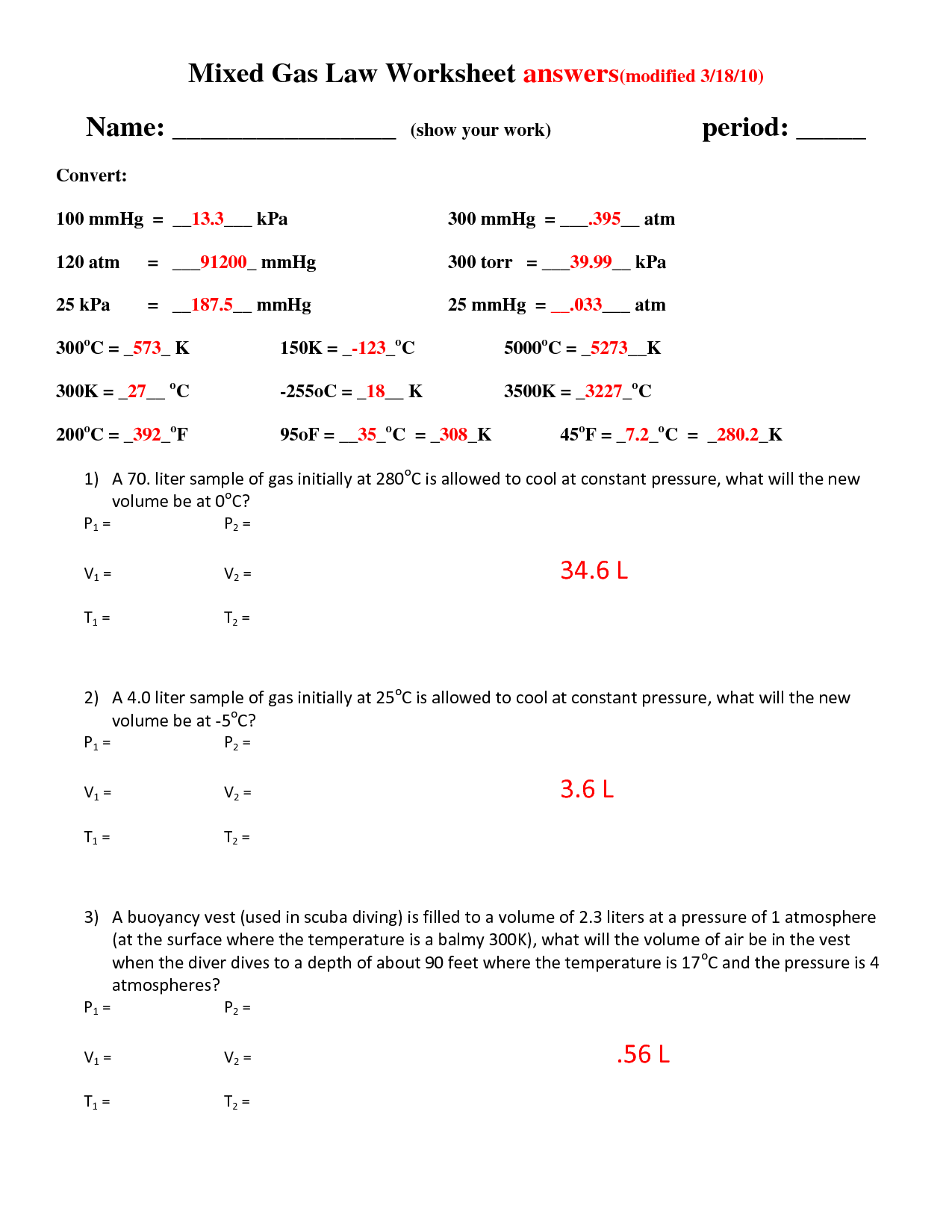
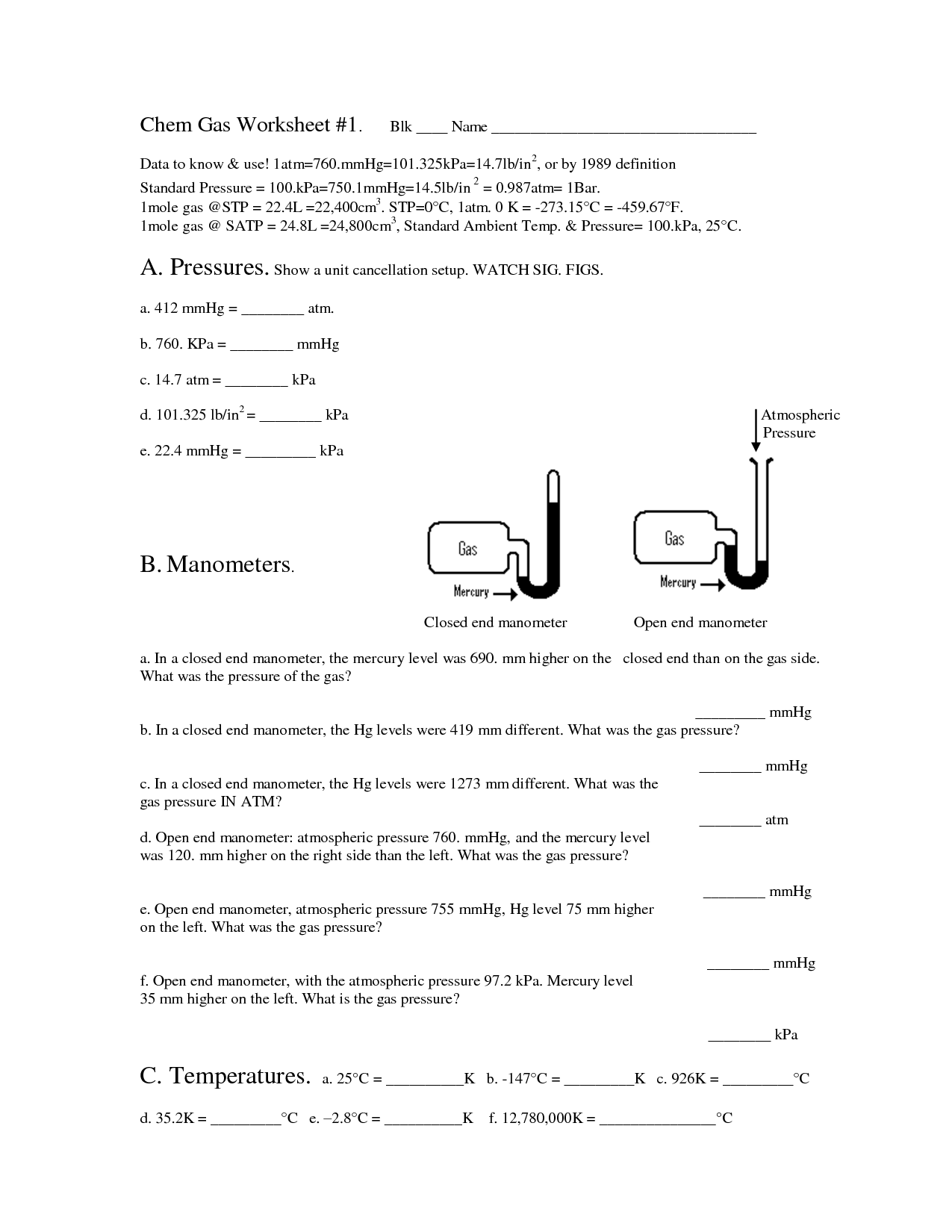
Gas Law Problems Worksheet with Answers
Gas law problems can be challenging, but with the right resources, you can tackle them with confidence. If you're a high school or college student studying chemistry or physics, you know that understanding and applying gas laws is essential. That's why having a gas law problems worksheet with answers can make all the difference in your learning experience. These worksheets provide a structured approach to practicing gas law calculations, ensuring you grasp the concepts and master problem-solving skills.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the ideal gas law and how is it derived?
The ideal gas law is a fundamental equation describing the behavior of an ideal gas, represented by the formula PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in Kelvin. It is derived from combining Boyle's law, Charles's law, and Avogadro's law, which relate pressure, volume, temperature, and number of moles for a gas. By combining these laws, the ideal gas law provides a simple and useful relationship between the four key variables that govern the behavior of an ideal gas.
How can the ideal gas law be used to calculate pressure?
The ideal gas law states that pressure (P) is directly proportional to the product of the number of moles of gas (n), the gas constant (R), and the temperature (T), and is inversely proportional to the volume (V) of the gas. By rearranging the ideal gas law equation (PV = nRT) to solve for pressure (P), one can calculate pressure by dividing the product of the number of moles, the gas constant, and the temperature by the volume of the gas: P = (nRT) / V. This equation allows you to calculate the pressure of an ideal gas when you have information on the number of moles of gas, the gas constant, the temperature, and the volume of the gas.
How do you calculate the volume of a gas using the ideal gas law?
To calculate the volume of a gas using the ideal gas law, you would rearrange the formula PV = nRT to solve for V, which represents volume. The formula would be V = nRT/P, where V is volume, n is the number of moles of gas, R is the ideal gas constant, T is the temperature in Kelvin, and P is the pressure. Plug in the values for n, R, T, and P to find the volume of the gas in the correct units, typically liters.
What is Avogadro's law and how does it relate to the ideal gas law?
Avogadro's law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This law helps to explain the behavior of ideal gases as it establishes a proportional relationship between the amount of a gas (in terms of moles) and its volume. When combined with Boyle’s, Charles’s, and Gay-Lussac's laws, Avogadro's law is used in the ideal gas law equation, which is PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature.
How can you calculate the number of moles of a gas using the ideal gas law?
To calculate the number of moles of a gas using the ideal gas law, you can rearrange the equation PV = nRT to solve for n, where n represents the number of moles. Simply divide the given pressure (P) multiplied by the volume (V) by the ideal gas constant (R) multiplied by the given temperature (T) in Kelvin. The resulting value will give you the number of moles of the gas.
What is the relationship between temperature and pressure in the ideal gas law?
According to the ideal gas law, temperature and pressure are directly proportional to each other when the volume and quantity of gas are constant. This means that as the temperature of a gas increases, the pressure it exerts also increases, and vice versa. This relationship is defined by the equation PV = nRT, where P is pressure, V is volume, n is number of moles, R is the ideal gas constant, and T is temperature in Kelvin.
How does the ideal gas law account for changes in temperature?
The ideal gas law, which is represented by the equation PV=nRT, accounts for changes in temperature by including the temperature term (T) in the formula. As temperature increases, the kinetic energy of gas molecules also increases, leading to a higher average velocity of the gas particles. This results in a higher pressure and volume of the gas according to the ideal gas law. Conversely, as temperature decreases, the kinetic energy and velocity of gas molecules decrease, leading to a decrease in pressure and volume. Therefore, the ideal gas law accounts for changes in temperature by acknowledging its direct impact on the pressure, volume, and quantity of gas in a system.
How can you calculate the temperature of a gas using the ideal gas law?
To calculate the temperature of a gas using the ideal gas law, you would need to rearrange the equation to solve for temperature. The ideal gas law is represented as PV = nRT, where P is pressure, V is volume, n is number of moles, R is the gas constant, and T is temperature in Kelvin. By rearranging the equation to solve for temperature, you can use the formula T = PV / nR. Simply plug in the values for pressure, volume, number of moles, and the gas constant to calculate the temperature of the gas in Kelvin.
How does the ideal gas law relate to real gases and deviations from ideal behavior?
The ideal gas law, PV = nRT, describes the behavior of ideal gases assuming they have negligible volume and no intermolecular forces. Real gases deviate from ideal behavior at high pressures or low temperatures, where intermolecular forces become significant. These deviations can be accounted for by introducing correction factors, such as the Van der Waals equation or compressibility factors, to better represent the behavior of real gases. Overall, the ideal gas law serves as a useful approximation for many gases under typical conditions but may require adjustments for more accurate predictions in real-world scenarios.
How can you use the ideal gas law to solve various gas law problems?
The ideal gas law, which is PV = nRT, can be used to solve various gas law problems by manipulating the equation to solve for the unknown variables, such as pressure (P), volume (V), temperature (T), or the amount of gas (n). By rearranging the equation and plugging in the known values, you can find the missing variable. This equation is particularly useful when dealing with gases at standard temperature and pressure conditions, or when changes in conditions are involved, such as pressure-volume work or heating and cooling processes.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
































Comments