Empirical Formula Worksheet
The empirical formula worksheet is a valuable tool for students studying chemistry. Designed to help comprehend the concept of empirical formulas and their calculations, this worksheet provides various exercises that allow students to practice and develop their skills in this area. By providing a clear and concise overview of the subject matter, the empirical formula worksheet offers an opportunity for students to solidify their understanding of empirical formulas and their real-world applications.
Table of Images 👆
- Percent Composition and Molecular Formula Worksheet
- Molecular and Empirical Formula Worksheet Answer Key
- Chemical Formula Writing Worksheet
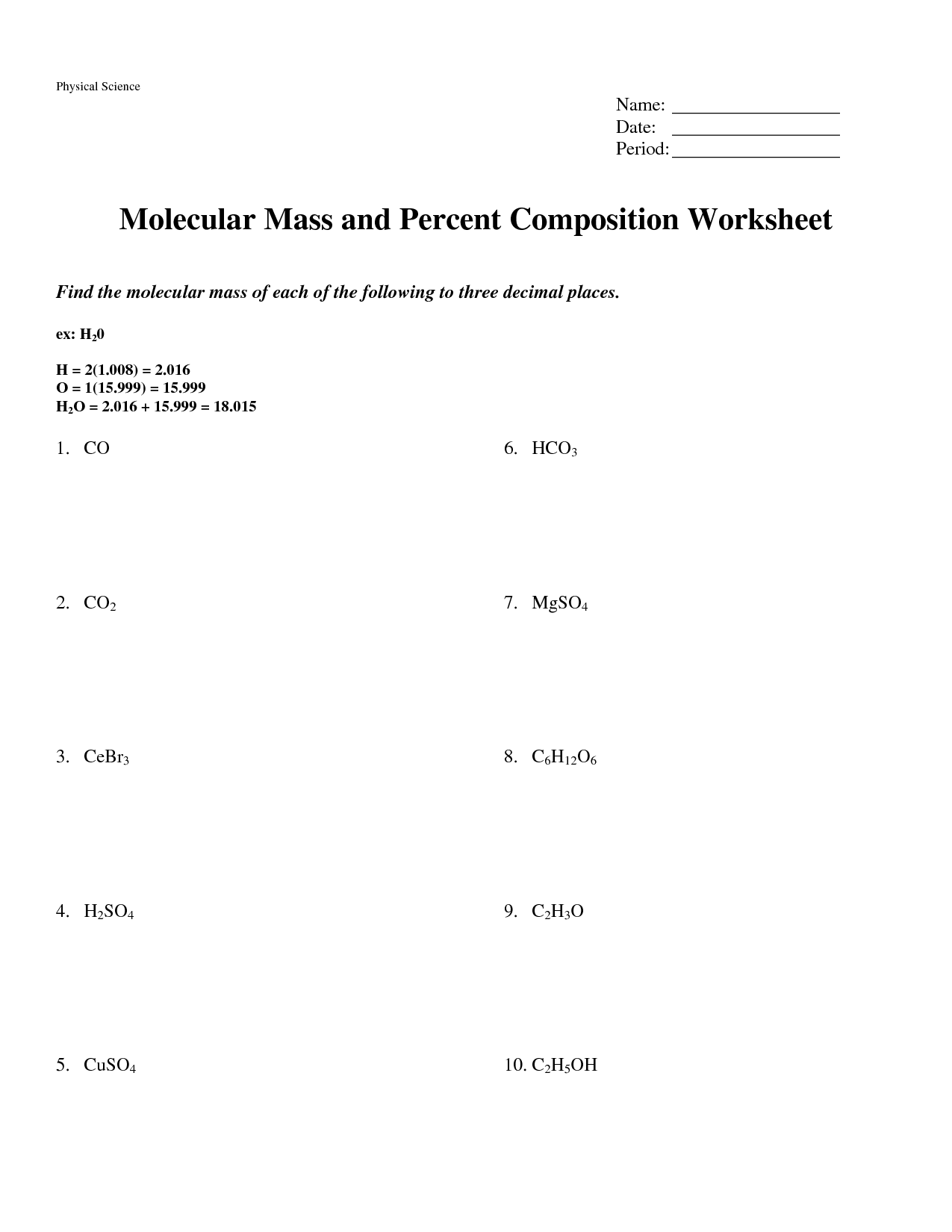
- Percent Composition and Molecular Mass Worksheet Answers
- Kingdom Animalia Phylum Porifera Sponges
- Ideal Gas Law Molecular Formula
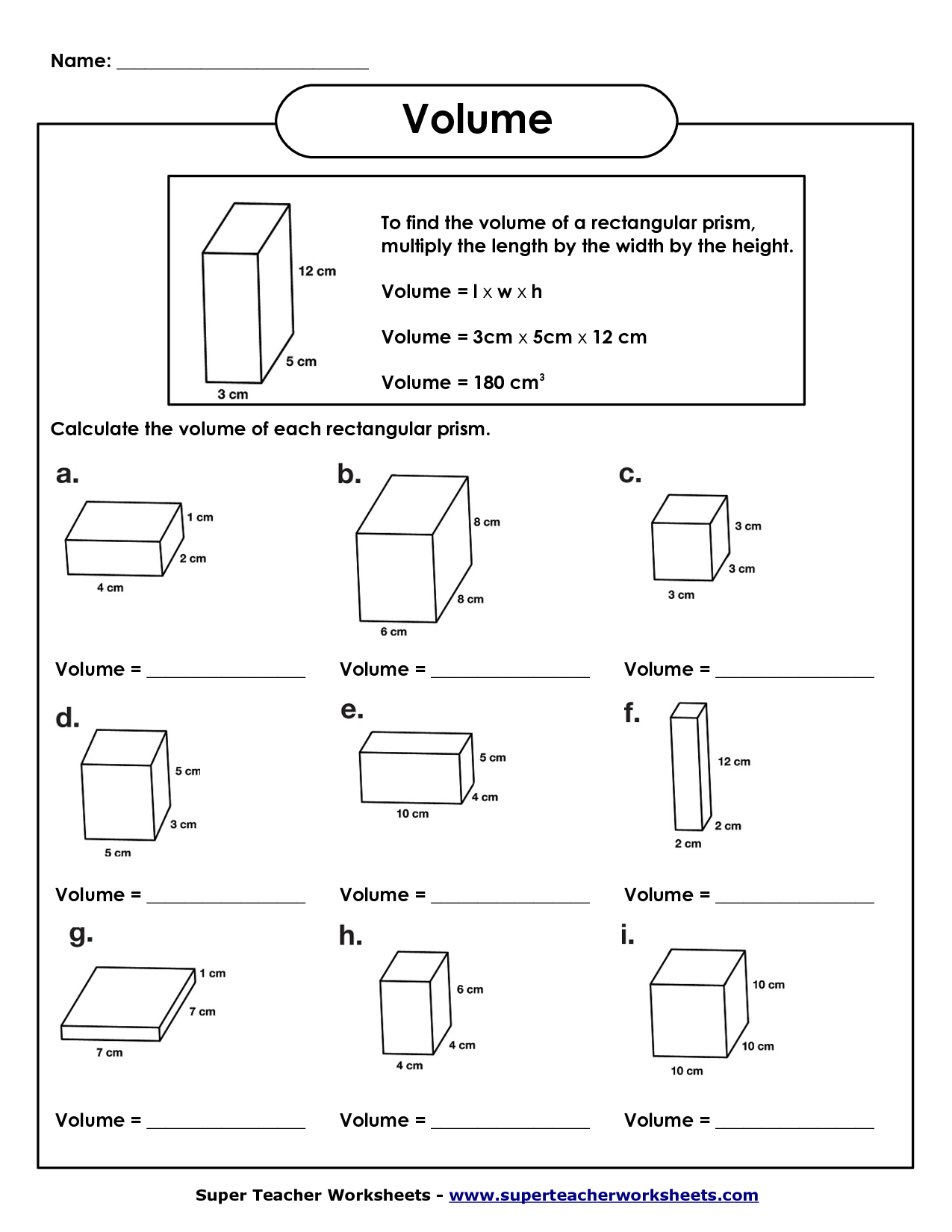
- Rectangular Prism Volume Worksheet
- Significant Figures Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the empirical formula?
The empirical formula is the simplest whole number ratio of atoms of each element in a compound, representing the composition of the compound.
How is the empirical formula different from the molecular formula?
The empirical formula represents the simplest whole number ratio of atoms in a compound, while the molecular formula gives the actual number of each type of atom in a molecule. The empirical formula is the reduced version of the molecular formula, which may be a multiple of the empirical formula. For example, the empirical formula for hydrogen peroxide is HO, while its molecular formula is H2O2.
How can the empirical formula be determined experimentally?
The empirical formula can be determined experimentally by analyzing the composition of a compound through techniques such as elemental analysis or mass spectrometry. By determining the mass percent of each element present in the compound, one can then convert these percentages to moles and find the simplest whole-number ratio of the elements, which represents the empirical formula of the compound.
What information is needed to calculate the empirical formula?
To calculate the empirical formula of a compound, you need the mass of each element present in the compound, either in grams or as a percentage, so that you can convert these values into moles. From there, you can determine the ratio of the different elements in the compound and simplify it to its simplest whole-number ratio to find the empirical formula.
Can the empirical formula be the same as the molecular formula?
Yes, the empirical formula can be the same as the molecular formula. This happens when the compound's molecular formula is the simplest whole number ratio of the elements present, which is also the empirical formula. In such cases, the molecular formula will be identical to the empirical formula, as there are no further whole-number ratios to simplify.
Can the empirical formula be different for different compounds?
Yes, the empirical formula can be different for different compounds. The empirical formula represents the simplest whole number ratio of atoms in a compound, so compounds with different types of atoms or different ratios of atoms will have different empirical formulas. It is important to note that compounds with different molecular structures can have the same empirical formula.
What are some examples of compounds with empirical formulas?
Some examples of compounds with empirical formulas are water (H2O), glucose (C6H12O6), hydrogen peroxide (H2O2), ammonia (NH3), methane (CH4), and table salt (NaCl).
How does the empirical formula relate to the elements present in a compound?
The empirical formula of a compound represents the simplest whole-number ratio of the elements present in that compound. It does not provide information about the actual number of atoms of each element. For example, the empirical formula for water is H2O, indicating that there are two hydrogen atoms for every one oxygen atom in the compound. This ratio gives a broad understanding of the composition of the compound in terms of its elements.
Can the empirical formula be simplified further?
The empirical formula represents the simplest whole number ratio of atoms in a compound and cannot be further simplified. It provides the relative number of atoms of each element in a compound, making it the most reduced form of the chemical formula.
How is the empirical formula useful in chemistry?
The empirical formula is useful in chemistry as it provides the simplest whole-number ratio of the elements in a compound, allowing chemists to determine the relative quantities of different elements present in a compound. This information is crucial for various applications, such as determining the stoichiometry of a reaction, calculating molar masses, and identifying unknown compounds based on elemental composition. By utilizing the empirical formula, chemists can make informed decisions about reaction conditions, understand the behavior of substances, and predict the properties of compounds.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments