Covalent Bonding Worksheets Middle School
Covalent bonding worksheets are a helpful tool for middle school students to solidify their understanding of this important scientific concept. These worksheets are designed to provide a clear and concise way for students to practice and reinforce their knowledge of covalent bonds, a type of chemical bond that occurs when two atoms share electrons. By offering a range of exercises and questions related to this subject, covalent bonding worksheets empower students to strengthen their foundational understanding and develop critical thinking skills in a topic that can often be challenging.
Table of Images 👆
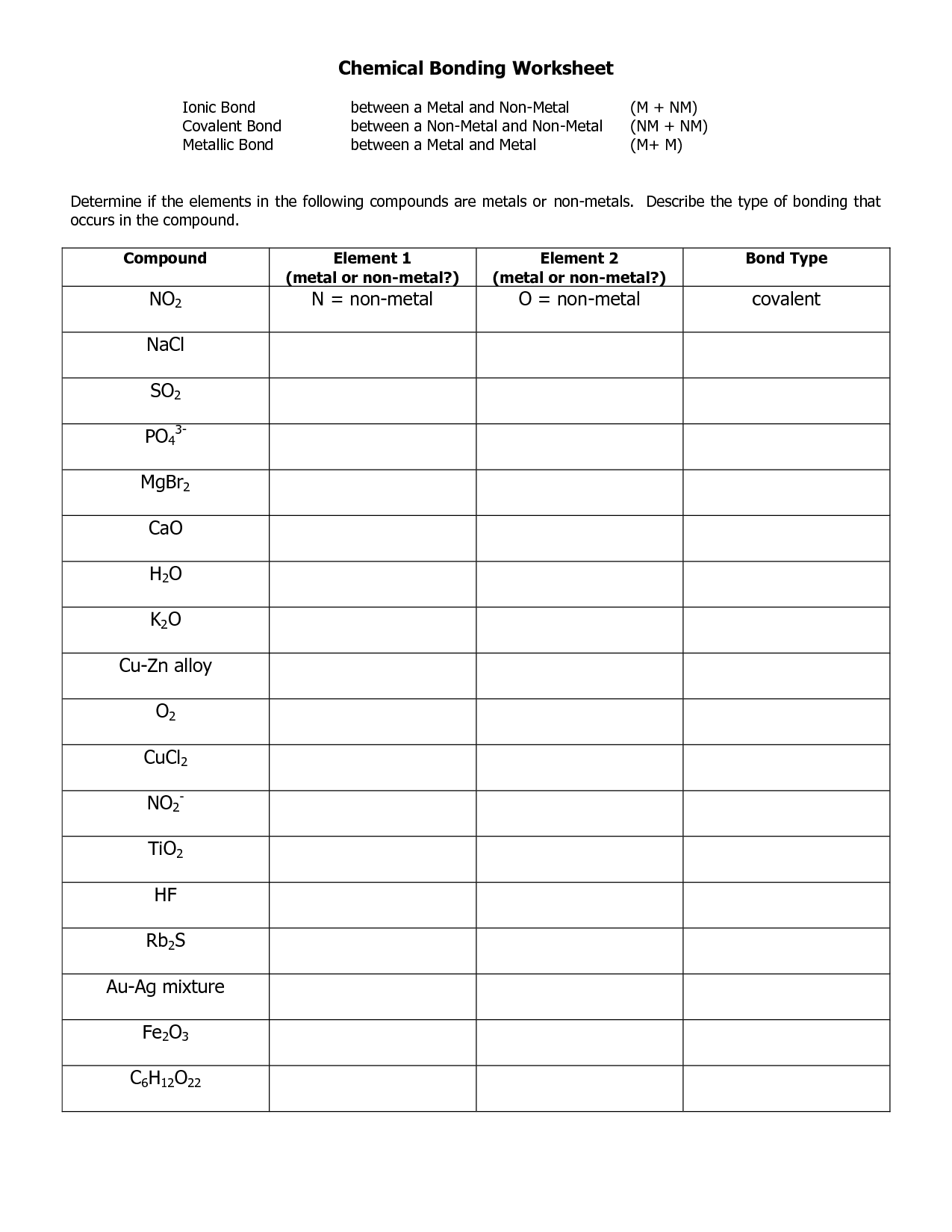
- Ionic and Covalent Bonding Worksheet
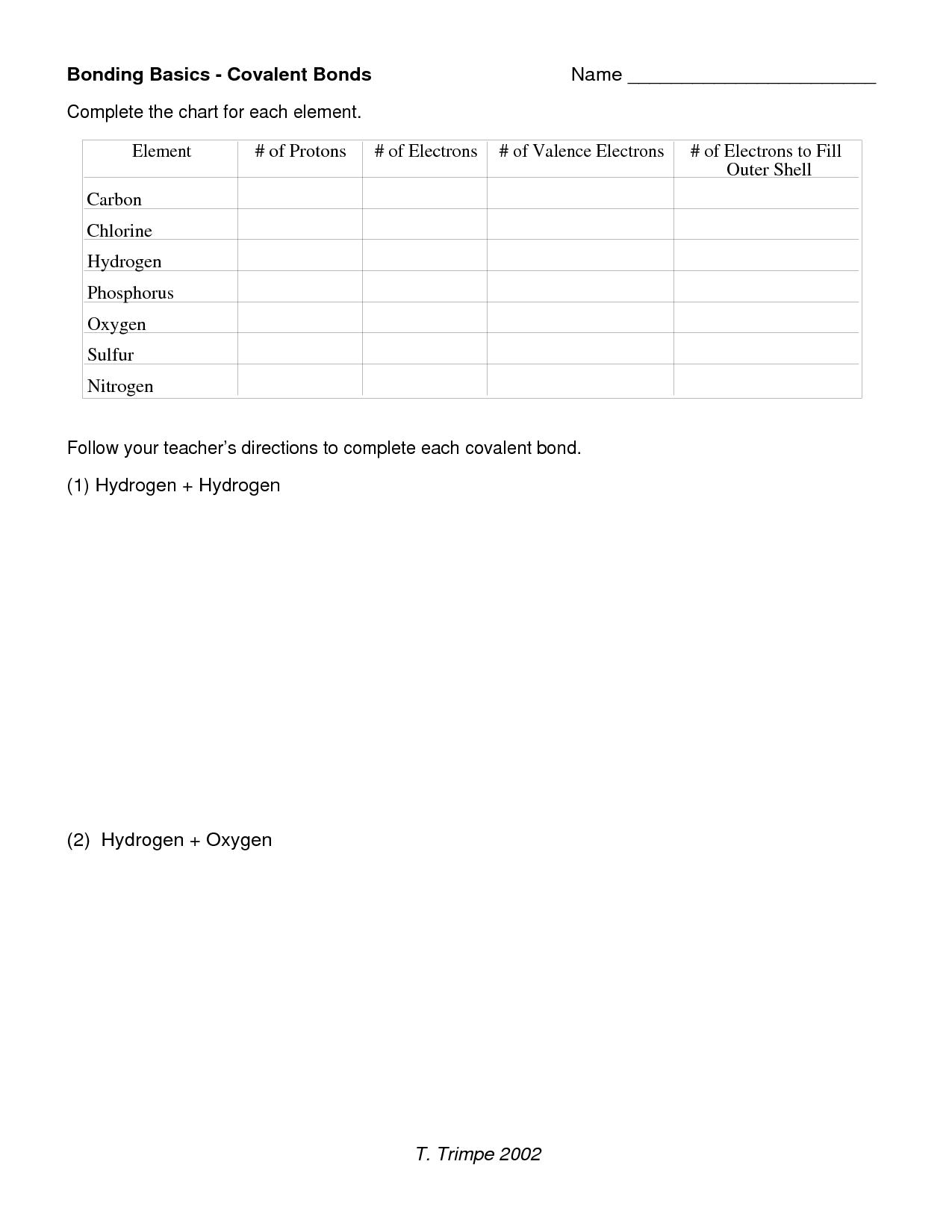
- Bonding Basics Covalent Bonds Worksheet Answers
- Ionic Bonding Dot Diagram and Worksheet
- Chemical Bonding Worksheet Answer Key
- Covalent Bonding Worksheet
- Ionic Bonding Worksheet Answer Key
- Chemical Bonds Worksheet Answers
- Practice Naming Ionic Compounds Worksheet Answers
- Ionic Covalent Bonding Worksheet
- Chapter 8 Covalent Bonding Worksheet Answers
- Naming Binary Ionic Compounds Worksheet Answers
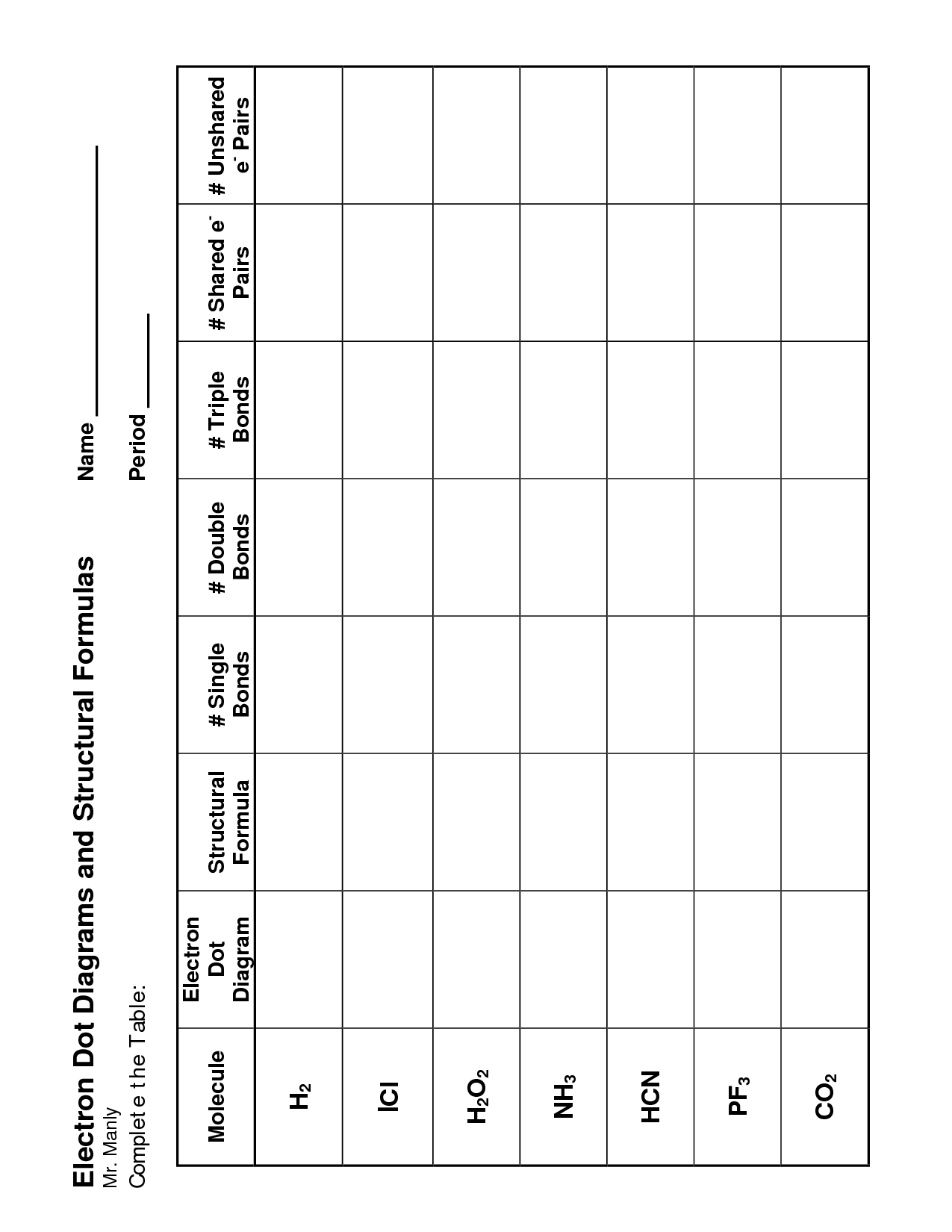
- Lewis Dot Structure Covalent Bond Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a covalent bond?
A covalent bond is a type of chemical bond where two atoms share a pair of electrons in order to achieve a stable configuration. This sharing of electrons allows the atoms to form a strong bond by filling their outer electron shell and increasing their stability. Covalent bonds are common in molecules and contribute to the formation of various compounds in nature.
How do atoms form covalent bonds?
Atoms form covalent bonds by sharing pairs of electrons between them. Each atom contributes one or more electrons to the bond, creating a shared electron pair that holds the atoms together. This sharing of electrons allows both atoms to achieve a more stable electron configuration, typically filling their outermost energy levels. The strength of the covalent bond is determined by the extent of electron sharing between the atoms.
What is the difference between a polar covalent bond and a nonpolar covalent bond?
In a polar covalent bond, electrons are unequally shared between atoms, resulting in a partial positive and partial negative charge on each atom. This occurs when there is a difference in electronegativity between the atoms. On the other hand, in a nonpolar covalent bond, electrons are equally shared between atoms, leading to no net charge separation. This typically happens when atoms have similar electronegativities.
How are Lewis dot structures used to represent covalent bonds?
Lewis dot structures are used to represent covalent bonds by illustrating the sharing of electrons between atoms in a molecule. Each atom's valence electrons are depicted as dots around the atomic symbol, and the bonds are shown as lines between the atoms, with each line representing a shared pair of electrons. This visual representation helps to understand the formation of covalent bonds and predict the molecular geometry and properties based on the arrangement of the atoms and electrons in the molecule.
How is the octet rule related to covalent bonding?
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with 8 electrons in their outermost shell. In covalent bonding, atoms share electrons to fill their outermost shell and achieve stability, typically resulting in the formation of molecules. By sharing electrons, atoms can satisfy the octet rule and form strong covalent bonds with other atoms in order to attain a more stable electron configuration.
What are some examples of molecules that are held together by covalent bonds?
Examples of molecules held together by covalent bonds include water (H2O), methane (CH4), carbon dioxide (CO2), ammonia (NH3), and hydrogen chloride (HCl). Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration, resulting in the creation of a molecular compound.
How do multiple covalent bonds between atoms affect the molecule's structure?
Multiple covalent bonds between atoms result in a more stable and rigid structure for the molecule. The additional bonds provide extra strength and hold the atoms in a specific arrangement, influencing the overall shape and geometry of the molecule. This can lead to increased complexity and diversity in the molecule's properties and interactions with other molecules.
What is the difference between a single covalent bond, a double covalent bond, and a triple covalent bond?
The main difference between a single covalent bond, a double covalent bond, and a triple covalent bond lies in the number of shared electron pairs between the bonded atoms. A single covalent bond involves the sharing of one pair of electrons between two atoms, while a double covalent bond involves the sharing of two pairs of electrons, and a triple covalent bond involves the sharing of three pairs of electrons. This results in varying bond strengths and bond lengths among the three types of covalent bonds.
How are electronegativity values used to determine the polarity of covalent bonds?
Electronegativity values are used to determine the polarity of covalent bonds by comparing the electronegativities of the two atoms involved in the bond. If there is a significant difference in electronegativity between the atoms, the bond is considered polar, with the more electronegative atom attracting the shared electrons more strongly and acquiring a partial negative charge, while the less electronegative atom becomes partially positive. The greater the electronegativity difference, the more polar the bond is. On the other hand, if the electronegativity values are similar, the bond is considered nonpolar, with equal sharing of electrons between the atoms.
How does the strength of a covalent bond compare to other types of chemical bonds?
Covalent bonds are generally stronger than other types of chemical bonds, such as ionic or hydrogen bonds. Covalent bonds involve the sharing of electrons between atoms, resulting in a strong connection between the atoms. In contrast, ionic bonds involve the transfer of electrons and are typically not as strong as covalent bonds. Hydrogen bonds are even weaker, formed between a hydrogen atom with a partial positive charge and a more electronegative atom with a partial negative charge. Overall, covalent bonds are typically the strongest type of chemical bond.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments