Compounds and Mixtures Worksheet Grade 8
Are you searching for a helpful educational resource to reinforce your understanding of compounds and mixtures? Look no further! This grade 8 worksheet is designed to effectively engage and challenge students with its comprehensive coverage of the topic. With a focus on entities and subjects related to compounds and mixtures, this worksheet is the perfect tool for students aiming to enhance their knowledge in this important area of science.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
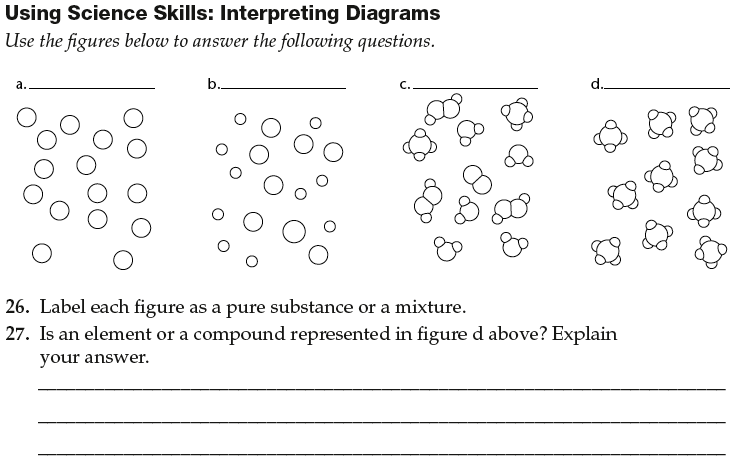
What is a compound?
A compound is a substance formed by the bonding of two or more elements in fixed proportions. These elements are chemically combined to create a new substance with unique properties different from those of its constituent elements.
How are compounds different from mixtures?
Compounds are made up of two or more different elements chemically bonded together in a fixed ratio, forming a new substance with unique properties, while mixtures are combinations of two or more substances that are physically mixed together and can be separated by physical means. Compounds have a specific chemical formula, while mixtures do not have a fixed composition and can vary in their proportions.
What is an element?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. Elements are the building blocks of all matter and are represented on the periodic table by their unique atomic number, symbol, and name. Each element consists of only one type of atom, which is characterized by a specific number of protons in its nucleus.
Give an example of a compound and describe its composition.
Water is an example of a compound composed of two hydrogen atoms bonded to one oxygen atom. This compound has a chemical formula of H2O, indicating the ratio in which the elements are combined. Water molecules consist of two hydrogen atoms covalently bonded to one oxygen atom, resulting in a polar molecule with properties critical for life such as high surface tension and the ability to dissolve many substances.
Explain the difference between a homogeneous mixture and a heterogeneous mixture.
A homogeneous mixture is uniform in composition, meaning its components are evenly distributed and not visibly distinguishable, like a solution of salt dissolved in water. In contrast, a heterogeneous mixture has uneven distribution of its components, making them visually distinguishable, such as a salad with various ingredients like lettuce, tomatoes, and croutons.
Provide an example of a homogeneous mixture and describe its characteristics.
An example of a homogeneous mixture is a solution of salt dissolved in water. In this mixture, the salt particles are uniformly distributed and appear to be the same throughout the entire solution. Homogeneous mixtures have properties such as a uniform composition, a single phase, and the components are not visually distinguishable from one another.
Give an example of a heterogeneous mixture and describe its characteristics.
A salad is an example of a heterogeneous mixture because it consists of various components that can be visually distinguished, such as lettuce, tomatoes, cucumbers, and dressing. Each component retains its individual properties and can be separated from the others. This mixture does not have a uniform composition, and the components are not evenly distributed throughout.
What are the methods used to separate mixtures?
Some common methods used to separate mixtures include filtration, distillation, evaporation, chromatography, and magnetism. Filtration involves passing a mixture through a porous barrier to separate components based on size. Distillation uses differences in boiling points to separate liquids. Evaporation involves evaporating the solvent to leave behind solid components. Chromatography separates components based on differences in solubility and affinity to a stationary phase. Magnetism can be used to separate magnetic components from non-magnetic ones by attracting the magnetic material to a magnet. These methods are used depending on the properties of the components in the mixture.
Describe the process of distillation and its application in separating mixtures.
Distillation is a process that involves heating a mixture to separate its components based on their boiling points. As the mixture is heated, the component with the lowest boiling point vaporizes first, rises, and then condenses back into liquid form in a separate container. This allows for the separation of different components in a mixture based on their boiling points. It is commonly used in various industries, such as in the production of alcoholic beverages, petroleum refining, and purifying water.
Why is it important to understand the difference between compounds and mixtures in various scientific fields?
Understanding the difference between compounds and mixtures is crucial in various scientific fields because it helps in accurately identifying and categorizing substances based on their chemical composition and properties. This knowledge is essential in fields such as chemistry, biology, environmental science, and materials science as it influences how these substances interact, react, and behave in different scenarios. Understanding compounds and mixtures also plays a significant role in research, experimentation, and application of scientific principles in real-world situations.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


































Comments