Classifying Chemical Reaction Types Worksheet
Chemical reactions can be complex and fascinating, but sometimes they can also be a bit daunting to understand. If you're a high school student or someone interested in deepening your knowledge of chemistry, our Classifying Chemical Reaction Types Worksheet is designed to help you grasp the essentials. This worksheet provides a clear and concise overview of different types of chemical reactions, allowing you to confidently identify the entities and subjects involved.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What are the different types of chemical reactions?
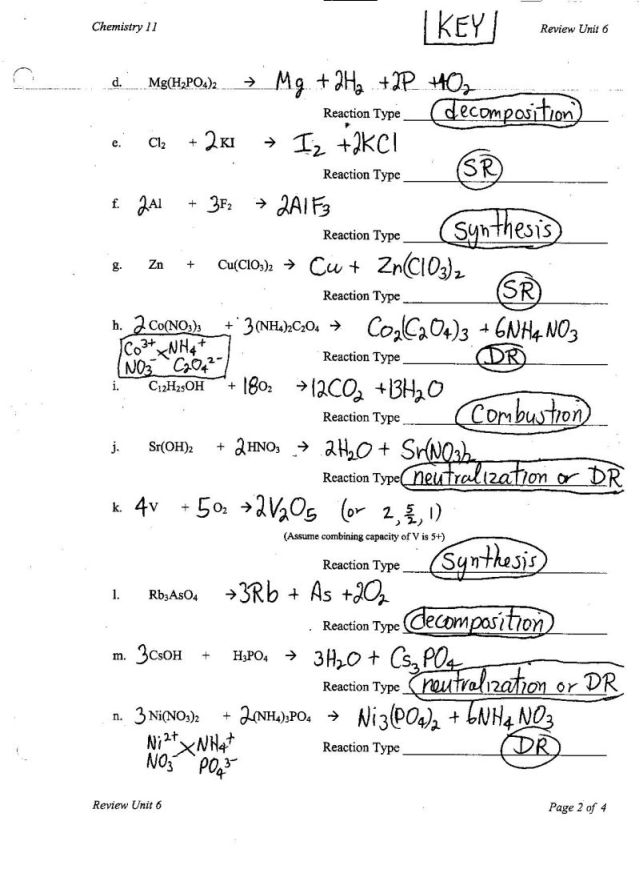
The different types of chemical reactions include synthesis (combination), decomposition, single displacement (replacement), double displacement (metathesis), combustion, and redox (oxidation-reduction) reactions. Each type of reaction involves different changes in chemical bonds and the rearrangement of atoms between reactants to form products.
How are synthesis reactions classified?
Synthesis reactions are classified based on the number of reactants involved. There are two main types of synthesis reactions: direct combination reactions, where two or more reactants combine to form a single product, and decomposition reactions, where a single reactant breaks down into two or more products.
How can decomposition reactions be categorized?
Decomposition reactions can be categorized into three main types based on the process and products involved: thermal decomposition, electrolytic decomposition, and photolytic decomposition. Thermal decomposition involves breaking down a compound into simpler substances through the application of heat. Electrolytic decomposition occurs through the passage of an electric current through a compound causing it to break down into its constituent elements or ions. Photolytic decomposition involves decomposition triggered by exposure to light, where a compound breaks down into simpler substances under the influence of photons.
What defines a combustion reaction?
A combustion reaction is a chemical reaction in which a substance reacts rapidly with oxygen, often releasing heat and light as products. It typically involves the burning of a fuel, such as hydrocarbons, to produce carbon dioxide and water as the main products. Combustion reactions are characterized by the presence of oxygen as a reactant and the generation of energy in the form of heat.
How are single replacement reactions identified?
Single replacement reactions can be identified by looking at the reactants and products of a chemical equation. In a single replacement reaction, an element replaces another element in a compound, resulting in a new compound and a different element. To identify a single replacement reaction, you should observe if one element in the reactants is replacing another element in the compound to form a new compound and a different element as a product.
What distinguishes double replacement reactions?
Double replacement reactions are characterized by the exchange of ions between two reactants, resulting in the formation of two new compounds. This type of reaction typically occurs in aqueous solutions and is driven by the formation of a precipitate, gas, or water. The key distinguishing feature of double replacement reactions is that the cations and anions of the reactants switch partners to form new compounds, while the overall charge balance is maintained.
What characterizes acid-base reactions?
Acid-base reactions are characterized by the transfer of protons (H+) from an acid to a base. Acids donate protons, leading to an increase in the H+ concentration, while bases accept protons, causing a decrease in the H+ concentration. These reactions result in the formation of water and a salt. The strength of an acid or base is determined by its tendency to donate (acid) or accept (base) protons.
How can precipitation reactions be recognized?
Precipitation reactions can be recognized by the formation of a solid, insoluble product when two solutions containing soluble compounds are mixed. Signs of a precipitation reaction include the appearance of a cloudy or turbid solution, the formation of a visible solid at the bottom of the container, or a change in color upon mixing the solutions. Additionally, the formation of a precipitate can be confirmed by conducting a filtration to separate the solid from the remaining solution.
What defines redox reactions?
Redox reactions, or reduction-oxidation reactions, are chemical reactions in which there is a transfer of electrons between reactants. In these reactions, one substance loses electrons (oxidation) while another gains electrons (reduction). The substance that gains electrons is called the oxidizing agent, while the substance that loses electrons is called the reducing agent. This transfer of electrons results in a change in the oxidation states of the atoms involved in the reaction.
How are exothermic and endothermic reactions classified?
Exothermic and endothermic reactions are classified based on the direction of heat flow during the reaction. Exothermic reactions release heat energy to the surroundings, resulting in an increase in temperature, while endothermic reactions absorb heat energy from the surroundings, leading to a decrease in temperature.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments