Charles and Boyle's Law Worksheet
Are you in need of a comprehensive worksheet to help you understand the concepts of Charles and Boyle's Law? Look no further! In this blog post, we will provide you with an entity-packed worksheet that is specifically designed to assist high school students in grasping the fundamentals of these important gas laws.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
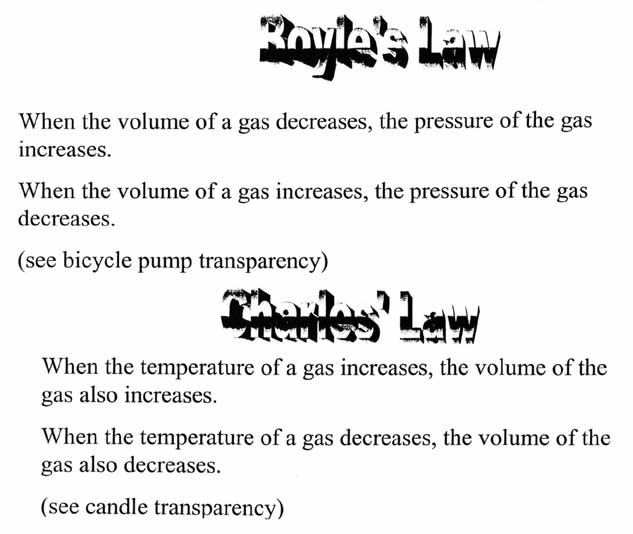
What is Charles' Law?
Charles' Law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure. This means that as the temperature of a gas increases, its volume also increases proportionally, and vice versa. This law is often summarized as V1/T1 = V2/T2, where V represents volume and T represents temperature in Kelvin.
What is Boyle's Law?
Boyle's Law states that the pressure of a gas is inversely proportional to its volume, when the temperature is held constant. In other words, if the volume of a gas increases, its pressure decreases and vice versa. This law helps to explain the relationship between pressure and volume in a closed system.
What are the key principles of Charles' Law?
Charles' Law states that, at constant pressure, the volume of a gas is directly proportional to its absolute temperature. This means that as the temperature of a gas increases, its volume also increases, and vice versa. The law can be represented mathematically as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature, respectively.
What are the key principles of Boyle's Law?
Boyle's Law states that the pressure of a gas is inversely proportional to its volume, given a constant temperature. This means that as the volume of a gas decreases, its pressure increases, and vice versa. The key principles of Boyle's Law can be summarized as: 1) pressure and volume are inversely related, 2) the temperature of the gas must remain constant, and 3) the relationship between pressure and volume is linear.
How does temperature relate to volume in Charles' Law?
According to Charles' Law, the volume of a gas is directly proportional to its temperature, given that pressure remains constant. This means that as the temperature of a gas increases, its volume will also increase, and as the temperature decreases, the volume will decrease. Essentially, Charles' Law states that if the pressure and amount of gas are constant, the volume of a gas will expand or contract in direct proportion to changes in temperature.
How does pressure relate to volume in Boyle's Law?
Boyle's Law states that the pressure of a gas is inversely proportional to its volume when the temperature is constant. This means that as the volume of a gas decreases, its pressure will increase, and vice versa. In other words, if you decrease the volume of a gas, the particles in the gas will collide more frequently with the container walls, leading to an increase in pressure. Conversely, if you increase the volume, the particles will have more space to move around and will collide less frequently, resulting in a lower pressure.
What is the mathematical equation for Charles' Law?
The mathematical equation for Charles' Law is V?/T? = V?/T?, where V? and T? represent the initial volume and temperature of a gas, and V? and T? represent the final volume and temperature, respectively.
What is the mathematical equation for Boyle's Law?
The mathematical equation for Boyle's Law is expressed as P1V1 = P2V2, where P1 and V1 represent the initial pressure and volume of a gas, while P2 and V2 represent the final pressure and volume of the gas when kept at a constant temperature.
How are Charles' Law and Boyle's Law related?
Charles' Law and Boyle's Law are related in that they both describe the behavior of gases under different conditions. Boyle's Law states that at constant temperature, the volume of a gas is inversely proportional to its pressure, meaning that as pressure increases, volume decreases, and vice versa. Charles' Law, on the other hand, states that at constant pressure, the volume of a gas is directly proportional to its temperature, meaning that as temperature increases, volume also increases, and vice versa. Together, these two laws provide a comprehensive understanding of how gases behave in different situations.
What are some practical applications of Charles' Law and Boyle's Law?
Charles' Law can be applied in areas such as refrigeration and air conditioning systems where changes in temperature result in changes in volume of gases. Boyle's Law is commonly used in scuba diving equipment to monitor changes in pressure as divers descend or ascend underwater, as well as in medical devices like ventilators to regulate the pressure of gases being delivered. Both laws are also utilized in the manufacturing of various products such as aerosol cans, where understanding the relationship between pressure, volume, and temperature is essential in producing reliable and safe products.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


































Comments