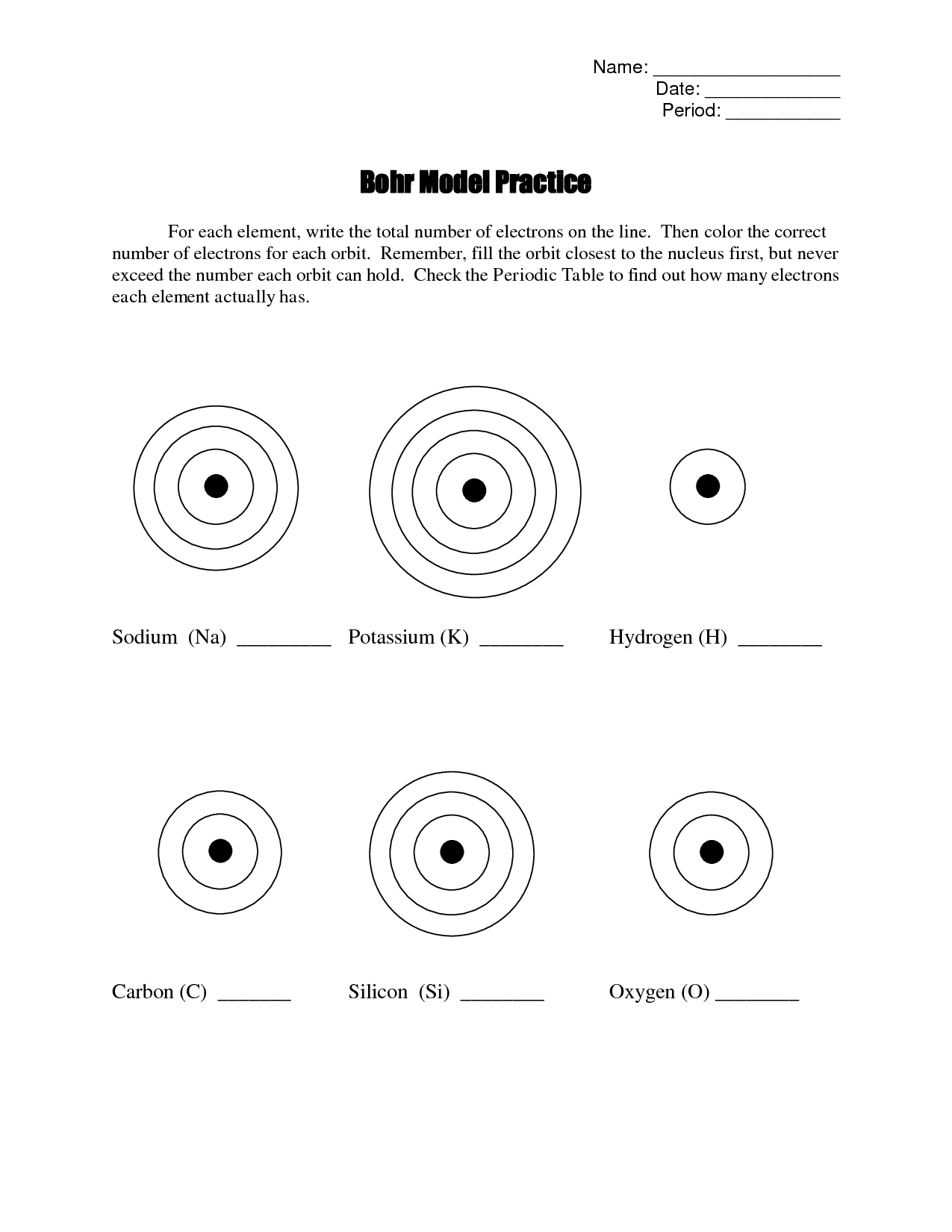
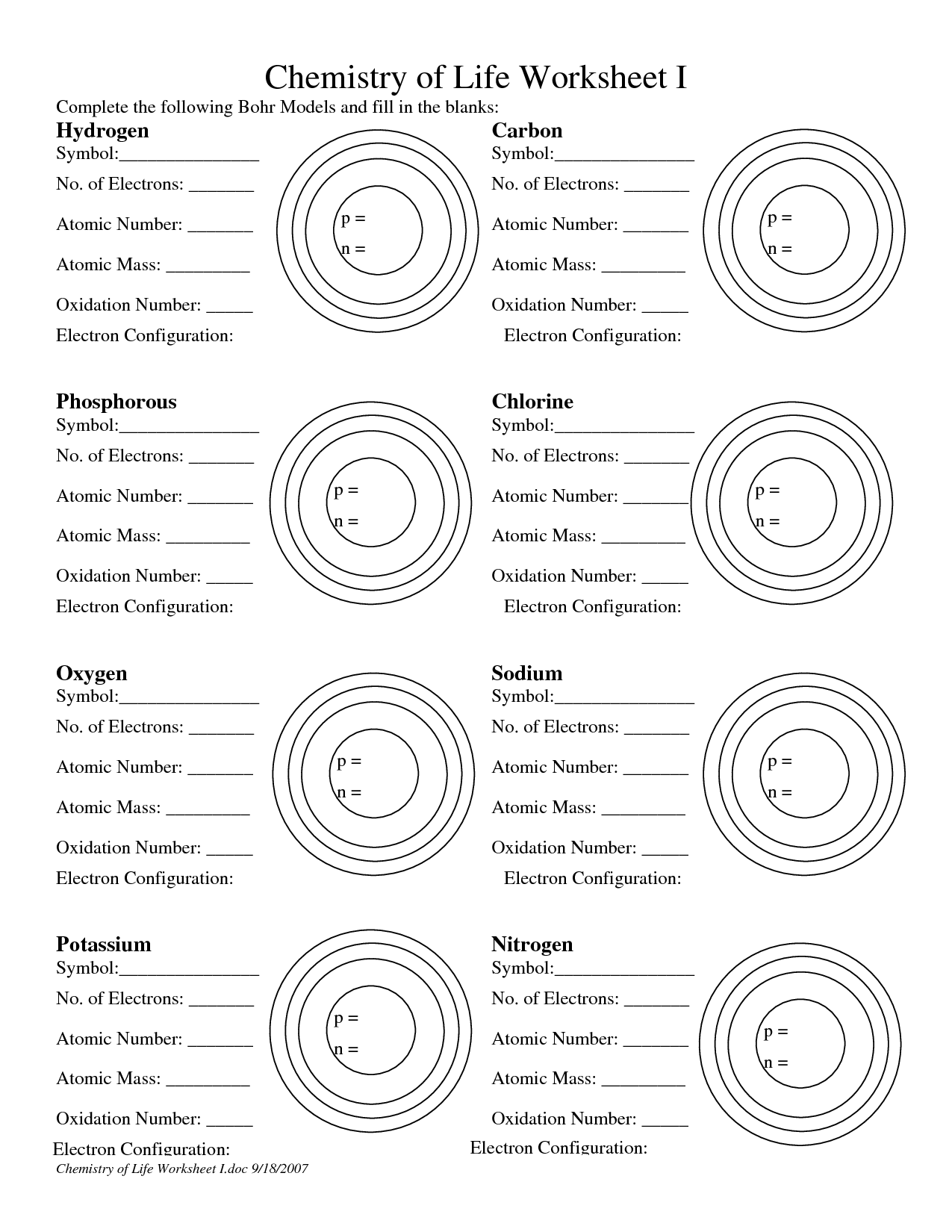
Bohr Model Practice Worksheet
Are you a student studying atomic structure and looking for a helpful resource to practice the Bohr model? Look no further! This blog post introduces a Bohr Model Practice Worksheet that will assist you in solidifying your understanding of this essential concept. With clearly labeled entities and subjects, this worksheet is specifically designed to cater to students in the field of chemistry or physics.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the Bohr model?
The Bohr model is a simplified model of the atom proposed by Niels Bohr in 1913. It suggests that electrons orbit the nucleus in specific energy levels or shells, rather than in continuous orbits. These shells have fixed energies, and electrons can only move between them by absorbing or emitting specific amounts of energy. The Bohr model helped to explain the stability of certain atoms and provided a framework for understanding atomic structure and the behavior of electrons.
Who developed the Bohr model?
The Bohr model was developed by Danish physicist Niels Bohr in 1913.
How does the Bohr model describe the structure of an atom?
The Bohr model describes the structure of an atom as having a central nucleus containing protons and neutrons, with electrons orbiting the nucleus in specific energy levels or shells. These electrons move in circular orbits at fixed distances from the nucleus, and each energy level can only hold a specific number of electrons. The model also states that electrons can jump between energy levels by absorbing or emitting energy in the form of photons.
What are the main features of the Bohr model?
The main features of the Bohr model include quantized electron energy levels, with electrons orbiting the nucleus in circular paths at specific distances known as shells. Electrons emit or absorb energy when transitioning between these energy levels, producing spectral lines. Additionally, the model suggests that electrons can only exist in certain stable orbits without radiating energy, and these orbits are characterized by specific angular momentum and quantized energy values.
What does the Bohr model propose about electron energy levels?
The Bohr model proposes that electrons orbit the nucleus in specific energy levels or orbits, and that these orbits are quantized, meaning electrons can only occupy certain allowed energy levels. These energy levels are determined by the electron's distance from the nucleus, with electrons in higher energy levels being further from the nucleus and having higher energy. The model also suggests that electrons can jump between these energy levels by absorbing or emitting energy in the form of photons.
How are electrons arranged in the Bohr model?
In the Bohr model, electrons are arranged in fixed orbits around the nucleus of an atom, similar to how planets orbit around the sun. Each orbit has a specific energy level, and electrons can only occupy these discrete energy levels. As electrons gain or lose energy, they can move between these orbits. This model helps to explain the stability and emission of certain wavelengths of light from atoms.
How does the Bohr model explain atomic spectra?
The Bohr model explains atomic spectra by proposing that electrons in an atom can only exist in specific energy levels or orbits. When an electron jumps between these levels, it either absorbs or emits energy in the form of light. The difference in energy levels determines the frequency and color of the light emitted or absorbed, which is observed as discrete lines in the atomic spectrum. This quantized nature of electron energy levels in the Bohr model accurately predicts the spectral lines seen in atomic emissions and supports the concept of quantized energy in atoms.
What is the significance of the Bohr model in understanding atomic structure?
The Bohr model of the atom was significant in understanding atomic structure as it was one of the first successful attempts to explain the behavior of electrons within an atom using quantum theory. By incorporating the concept of quantized electron energy levels and the emission of discrete spectral lines, the Bohr model provided a framework for understanding how electrons move in specific orbits around the nucleus. This model laid the foundation for our modern understanding of atomic structure and led to later developments in quantum mechanics.
How does the Bohr model relate to the modern understanding of atoms?
The Bohr model of the atom, proposed by Niels Bohr in 1913, introduced the concept of quantized energy levels for electrons orbiting the nucleus. While this model was a significant advancement in understanding atomic structure, it was later refined by the development of quantum mechanics. The modern understanding of atoms incorporates quantum mechanics, which describes the behavior of electrons as both particles and waves and provides a more accurate depiction of atomic behavior than the Bohr model. Despite its limitations, the Bohr model laid the foundation for further advances in atomic theory and continues to be a key concept in the study of atomic structure.
What are the limitations of the Bohr model?
The Bohr model of the atom has limitations such as being unable to explain the spectra of multi-electron atoms, the inability to account for the relative intensities of spectral lines, and it doesn't consider the wave-particle duality of electrons. Additionally, the model contradicts the uncertainty principle and doesn't accurately predict the transition of electrons between energy levels.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
































Comments