Bohr Model Atomic Structure Worksheet
A Bohr Model Atomic Structure Worksheet is a valuable educational tool designed to help students understand the concept of atomic structure and the arrangement of electrons in an atom. This worksheet is suitable for students who are studying chemistry or physics and are looking to solidify their understanding of the Bohr model. By providing clear instructions and relevant questions, this worksheet enables students to explore the entity and subject of atomic structure in a structured and comprehensive manner.
Table of Images 👆
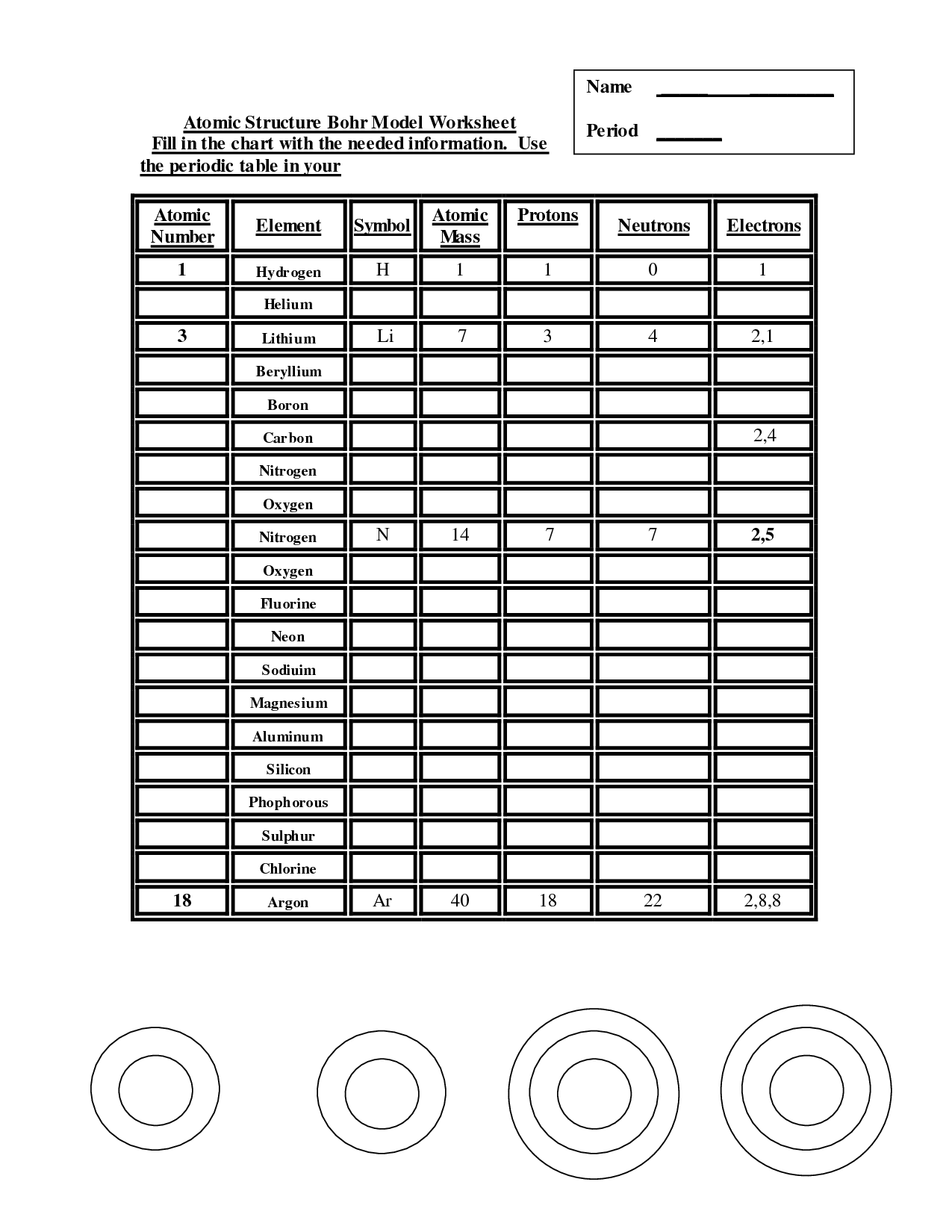
- Atomic Structure Bohr Model Worksheet
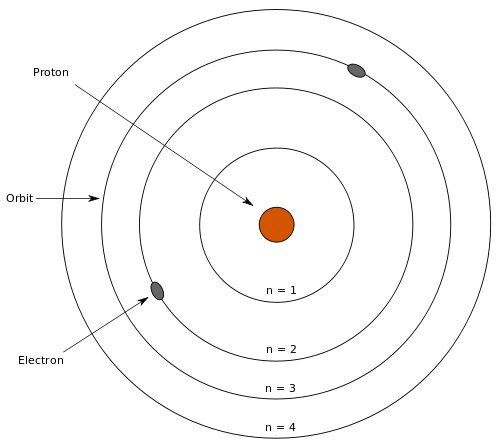
- Blank Bohr Model Diagram
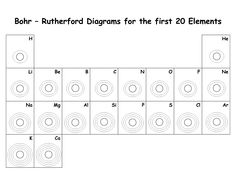
- Diagram of the First 20 Elements Bohr Model
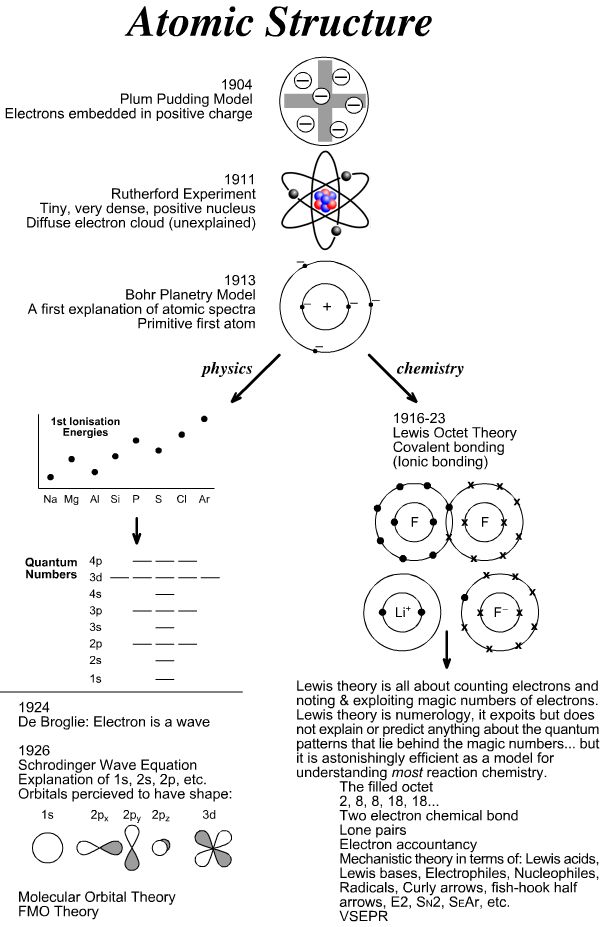
- Atomic Structure Model Timeline
- Basic Atomic Structure Worksheet Answer Key
- Institute of Theoretical Physics at the University of Copenhagen
- Electron Configuration Worksheet Answer Key
- John Thomson Atomic Theory
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the Bohr model of atomic structure?
The Bohr model of atomic structure, proposed by Niels Bohr in 1913, describes the atom as a small, positively charged nucleus surrounded by electrons that move in circular orbits at specific energy levels. These energy levels are quantized, meaning electrons can only occupy certain orbits with specific energy values. The model also explains the emission and absorption of light by electrons transitioning between energy levels, with the difference in energy levels corresponding to the frequency of the emitted or absorbed light.
Who developed the Bohr model?
The Bohr model was developed by Danish physicist Niels Bohr in 1913.
How does the Bohr model explain the stability of an atom?
The Bohr model explains the stability of an atom by proposing that electrons orbit the nucleus in specific energy levels or shells. Electrons in these energy levels have fixed orbits and do not radiate energy as long as they remain in these stable orbits. This stable configuration prevents the electrons from spiraling into the nucleus and helps maintain the overall stability of the atom.
What is meant by the term "quantized energy levels" in the Bohr model?
In the Bohr model of the atom, "quantized energy levels" refer to the specific, discrete energy values that electrons can possess when they are in their stable orbits around the nucleus. These energy levels are restricted to certain values and transitions between them result in the emission or absorption of electromagnetic radiation, giving rise to distinct spectral lines in an atom's atomic spectrum. The quantization of energy levels is a key concept in understanding the behavior of electrons in atoms according to the principles of quantum mechanics.
How does the Bohr model explain the emission and absorption of light by atoms?
The Bohr model explains the emission and absorption of light by atoms through changes in the energy levels of electrons. When an electron jumps from a higher energy level to a lower one, it emits a photon of a specific wavelength, resulting in the emission of light. Conversely, when an electron absorbs a photon, it moves to a higher energy level. This model helps to understand the discrete spectrum of light emitted and absorbed by atoms, as each electron transition corresponds to a specific photon energy level.
What are the limitations of the Bohr model?
The Bohr model of the atom has limitations as it is unable to explain more complex elements beyond hydrogen or singly ionized helium. It also does not account for electron-electron interactions, which play a significant role in the behavior of multiple electron systems. Additionally, the model does not provide a clear explanation for the spectral lines of atoms with more than one electron. Thus, while the Bohr model was revolutionary in understanding atomic structure, it has certain limitations when it comes to explaining more complex atomic systems.
How does the Bohr model relate to the concept of electron shells or energy levels?
The Bohr model of the atom introduced the concept of electron shells or energy levels, which are specific orbits where electrons can reside around the nucleus. In the Bohr model, electrons are confined to discrete energy levels, and each shell corresponds to a specific energy level further away from the nucleus. This model helped to explain the stability of certain elements and the emission of specific spectral lines, laying the foundation for our current understanding of atomic structure.
What is the significance of the "ground state" in the Bohr model?
The ground state in the Bohr model of the atom is the lowest energy state that an electron can occupy in an atom. It is significant because it is the most stable configuration for an atom, with the electron being as close to the nucleus as possible. This state represents the point at which the electron has the least amount of energy and is the basis for understanding the energy levels and the emission or absorption of energy in atoms.
How does the Bohr model explain the movement of electrons within an atom?
The Bohr model explains the movement of electrons within an atom by proposing that electrons orbit the nucleus in fixed, circular paths at specific energy levels. These energy levels are quantized, meaning the electrons can only occupy certain orbits with defined energies. Electrons can jump between these energy levels by absorbing or emitting energy in the form of photons. This model provides a simple explanation for how electrons move within an atom and helps to describe the structure of atoms and their spectral lines.
What is the connection between the Bohr model and the development of quantum mechanics?
The Bohr model of the atom, proposed by Niels Bohr in 1913, laid the foundation for the development of quantum mechanics by introducing the concept of quantized energy levels in atoms. This idea challenged classical physics and led to the exploration of new theories to explain the behavior of matter at the atomic and subatomic level. The Bohr model was a stepping stone towards the creation of quantum mechanics, which revolutionized our understanding of the behavior of particles and energy, ultimately leading to the modern quantum theory.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




















Comments