Basic Atomic Structure Worksheet Answer Key
Are you a high school student or teacher searching for a reliable answer key to accompany your basic atomic structure worksheet? Look no further! In this blog post, we have provided a comprehensive answer key that covers all the essential concepts and questions related to atomic structure. Whether you are studying chemistry for the first time or reviewing the subject, our answer key will ensure you have a solid understanding of this fundamental topic.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is an atom? - The basic unit of matter, consisting of a nucleus and electrons.
An atom is the basic unit of matter, consisting of a nucleus that contains protons and neutrons, surrounded by electrons that orbit the nucleus in defined energy levels.
What is the nucleus of an atom? - The central part of the atom, composed of protons and neutrons.
The nucleus of an atom is the central part of the atom that contains protons and neutrons.
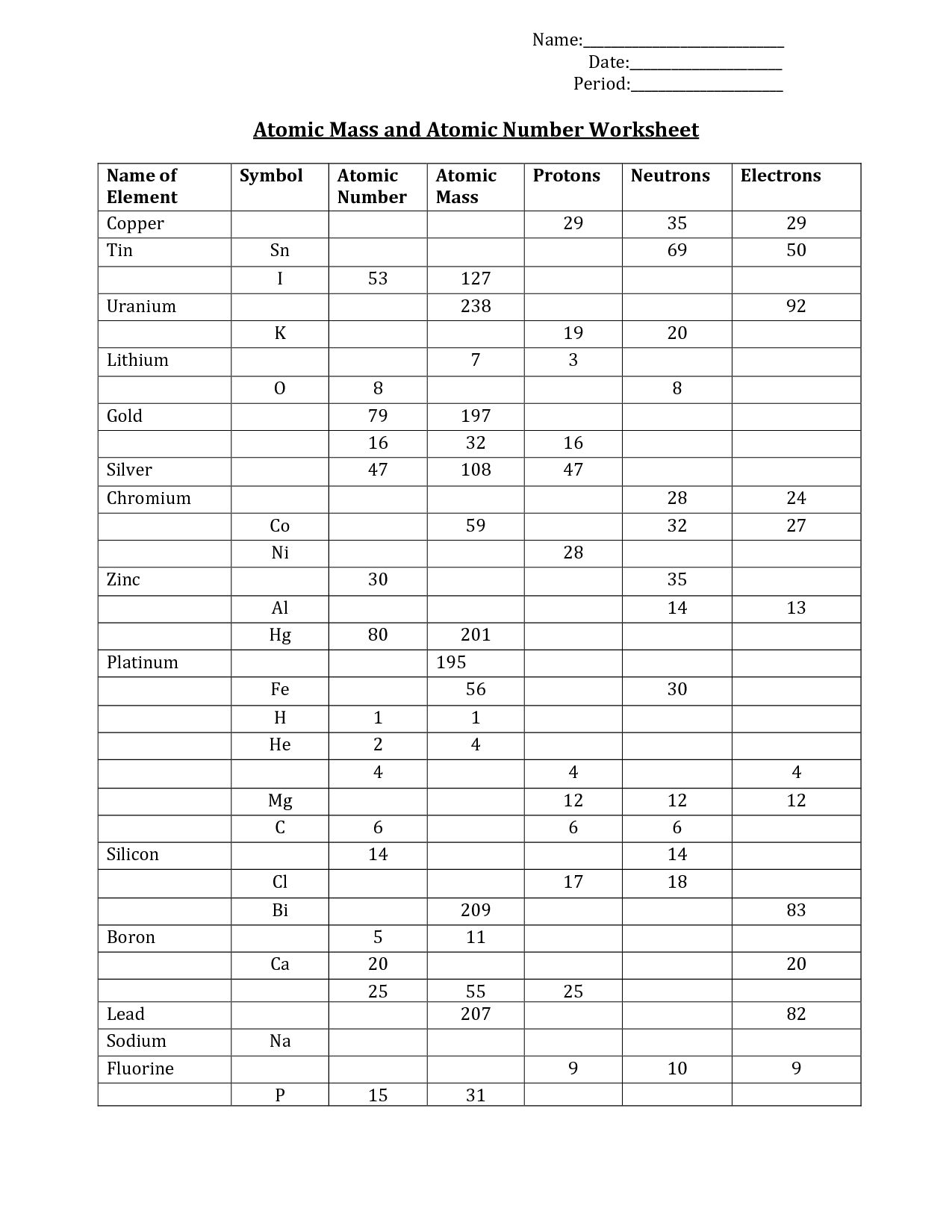
What are protons? - Positively charged particles found in the nucleus of an atom.
Protons are positively charged particles found in the nucleus of an atom.
What are neutrons? - Neutral particles found in the nucleus of an atom.
Neutrons are neutral particles found in the nucleus of an atom. They have no electrical charge, and along with protons, they make up the nucleus of an atom. Neutrons play a crucial role in determining the stability and properties of an atom.
What are electrons? - Negatively charged particles that orbit around the nucleus of an atom.
Electrons are negatively charged fundamental particles that orbit around the nucleus of an atom in specific energy levels, contributing to the atom's overall charge and reactivity.
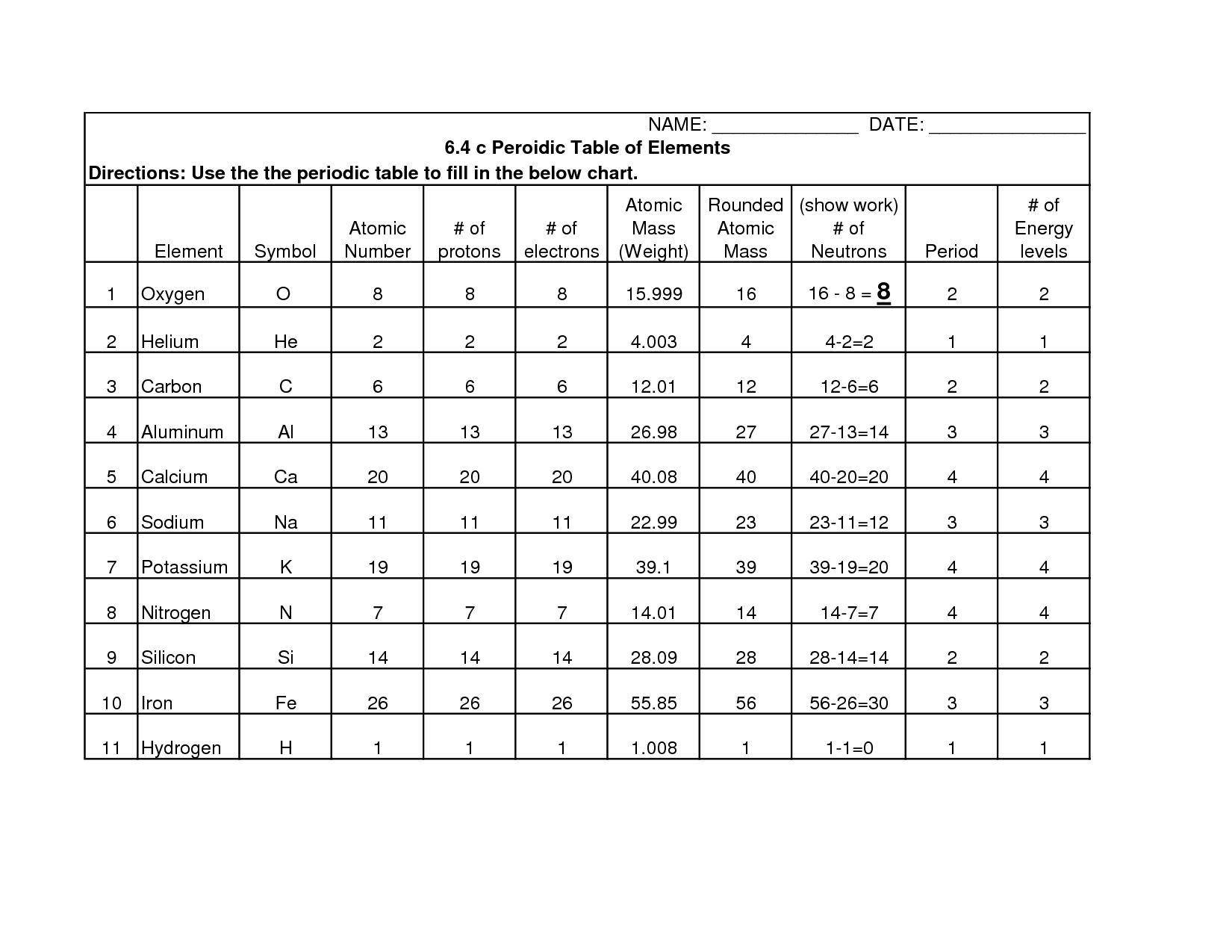
What is an atomic number? - The number of protons in an atom, which determines its element.
The atomic number is the number of protons in an atom, which determines its element.
What is an atomic mass? - The combined mass of the protons and neutrons in an atom.
The atomic mass is the combined mass of the protons and neutrons in an atom.
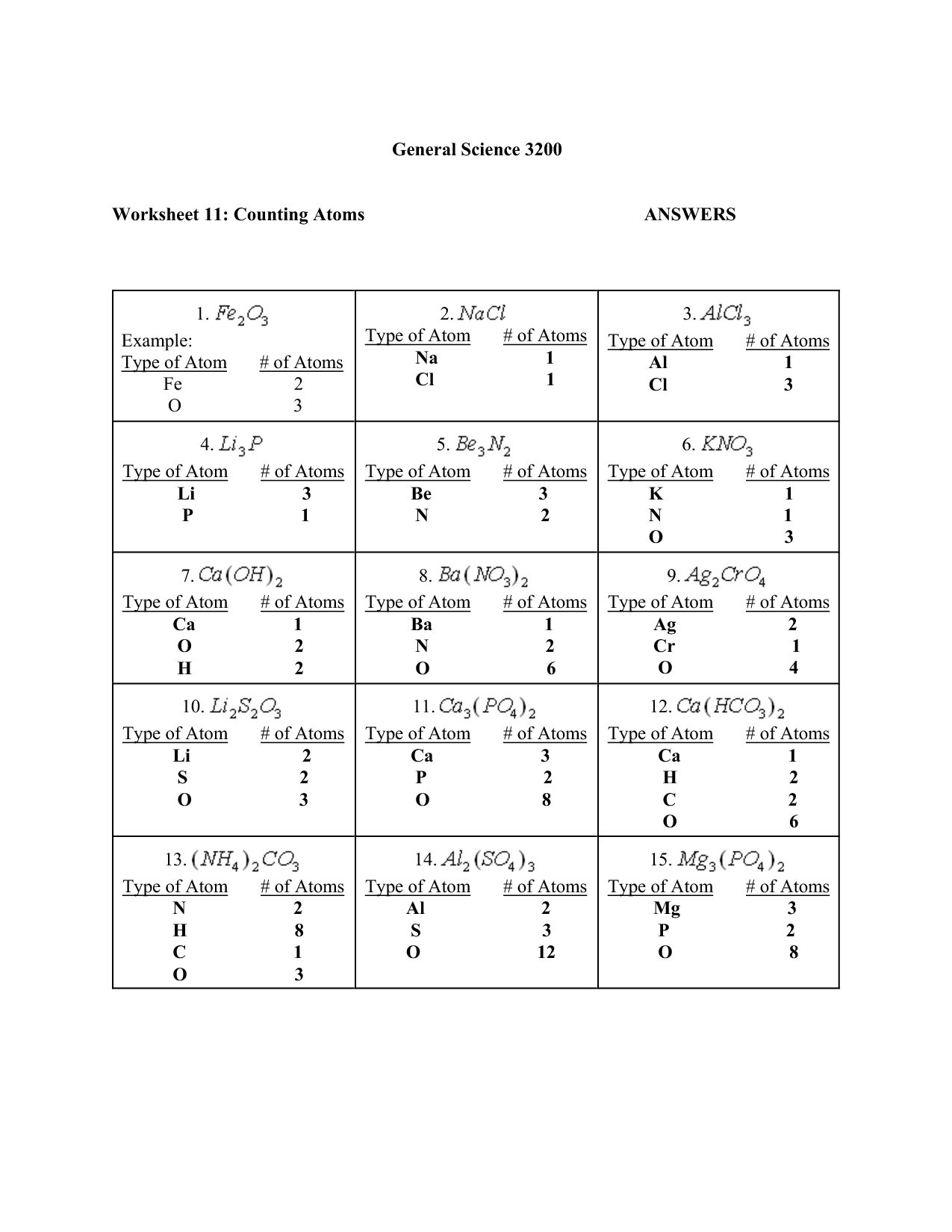
What are isotopes? - Atoms of the same element with different numbers of neutrons.
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons.
What is the Bohr model? - A model of the atom where electrons are depicted as orbiting in specific energy levels.
Correct! The Bohr model is a simplified representation of the atom that shows electrons orbiting the nucleus in distinct energy levels or shells. This model was proposed by Niels Bohr in 1913 and was a significant advancement in understanding the structure of the atom.
What is the electron configuration? - The arrangement of electrons in the energy levels or orbitals around the nucleus.
The electron configuration is the arrangement of electrons in the energy levels or orbitals around the nucleus of an atom. It describes how many electrons are in each energy level or orbital, following the rules of quantum mechanics and the Pauli exclusion principle.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.































Comments