Balancing Equations Worksheet Key
A balancing equations worksheet is an essential tool for students learning chemistry or physics concepts. Designed to reinforce understanding and improve problem-solving skills, these worksheets provide exercises that allow students to practice balancing chemical equations and equations related to physical laws. By focusing on the fundamental concepts of entities and subjects within equations, these worksheets help students grasp the importance of maintaining equilibrium and accurately representing the relationships between various elements.
Table of Images 👆
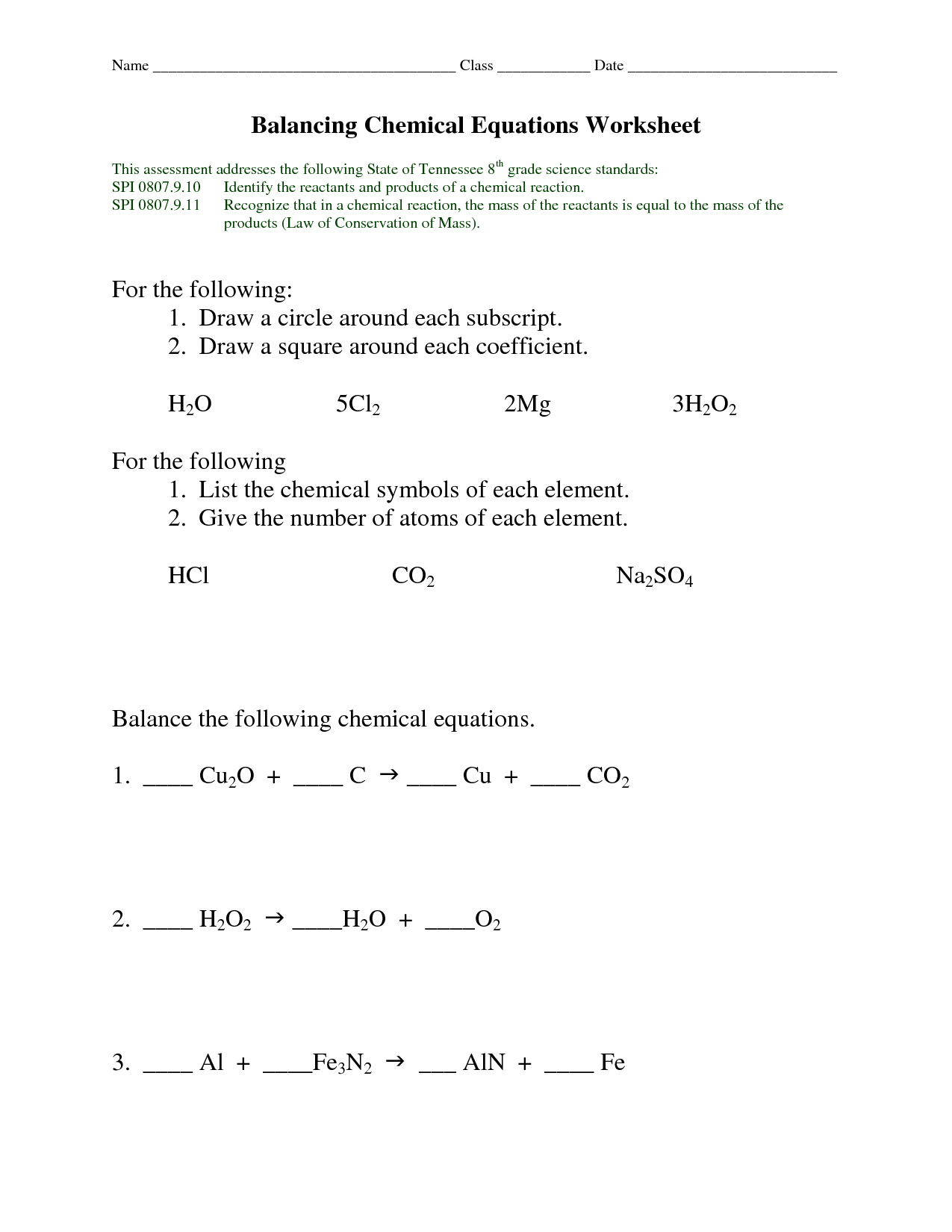
- Balancing Chemical Equations Worksheet Answer Key
- Writing Chemical Equations Worksheet Answers
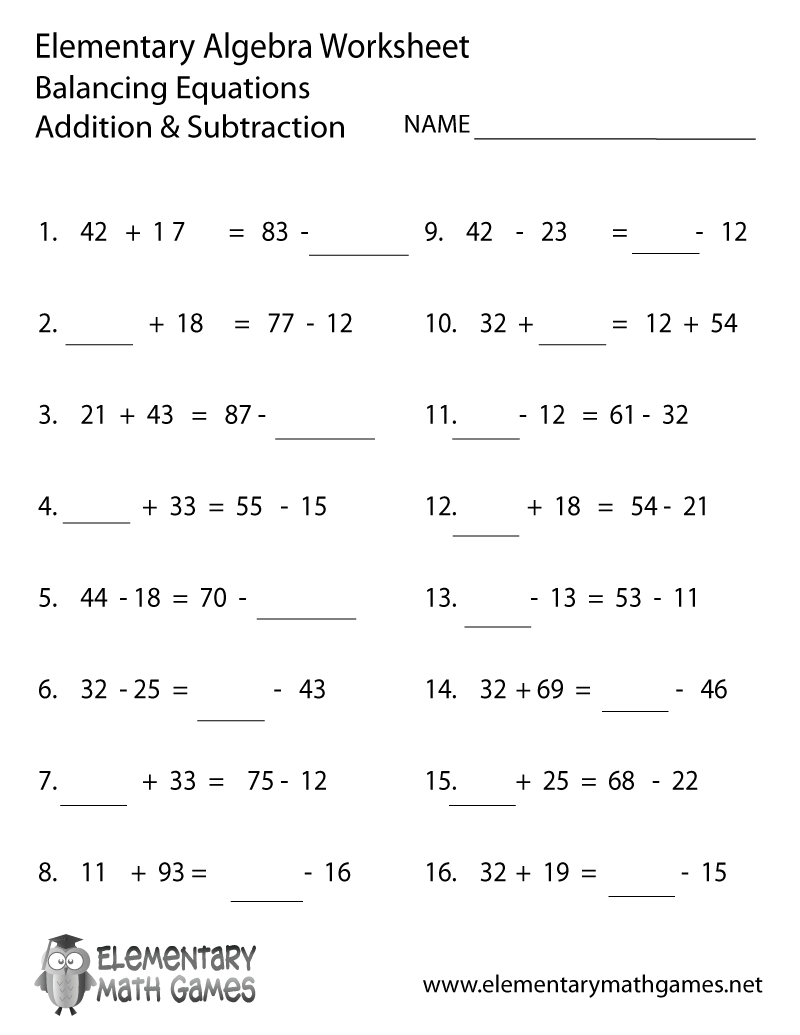
- Free Addition and Subtraction Worksheet
- Balancing Chemical Equations Worksheet Answers
- Types Chemical Reactions Worksheets Answers
- Balancing Redox Reactions Worksheet Answers
- Balancing Equations Worksheet Answer Key
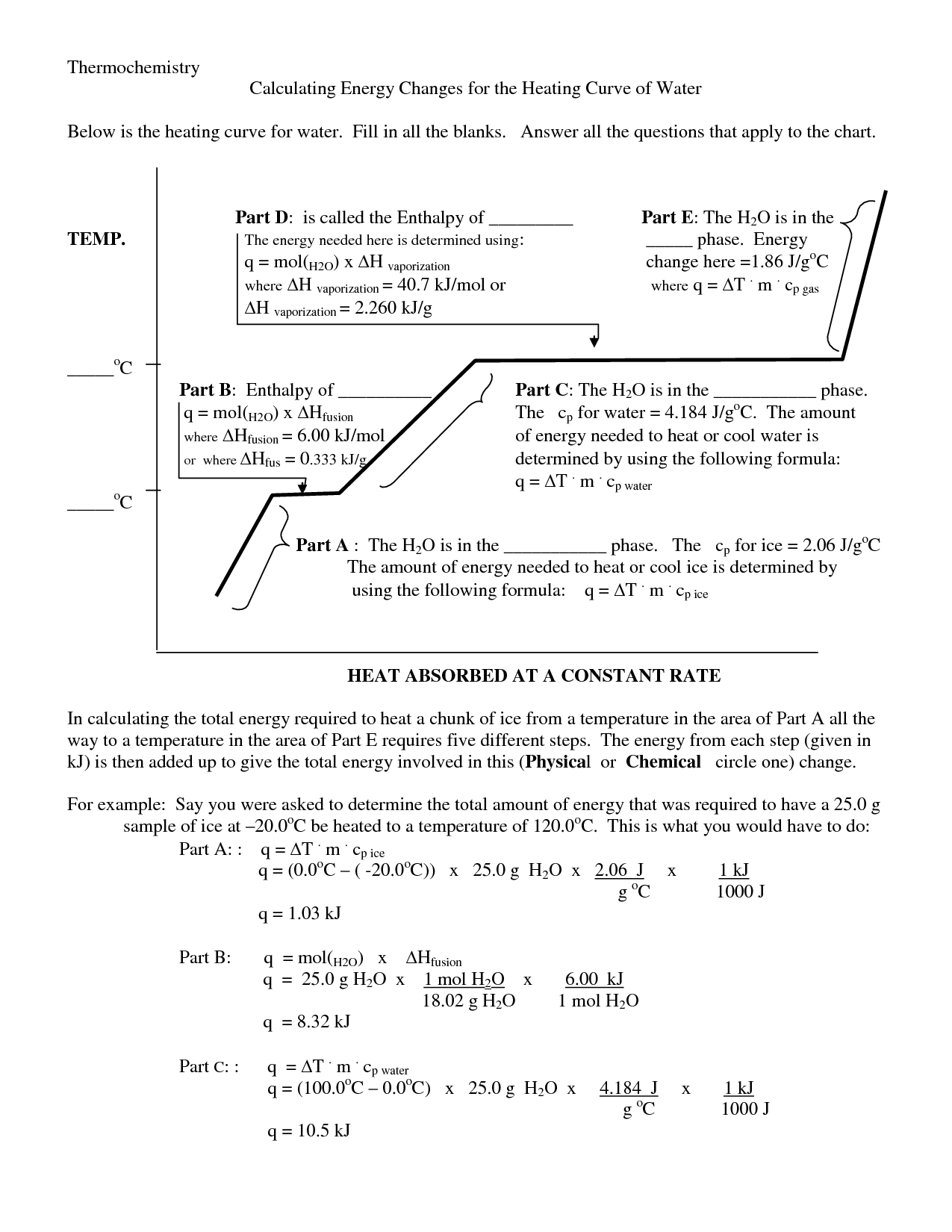
- Heating Curve Kinetic and Potential Energy
- Balancing Chemical Equations Worksheet Middle School
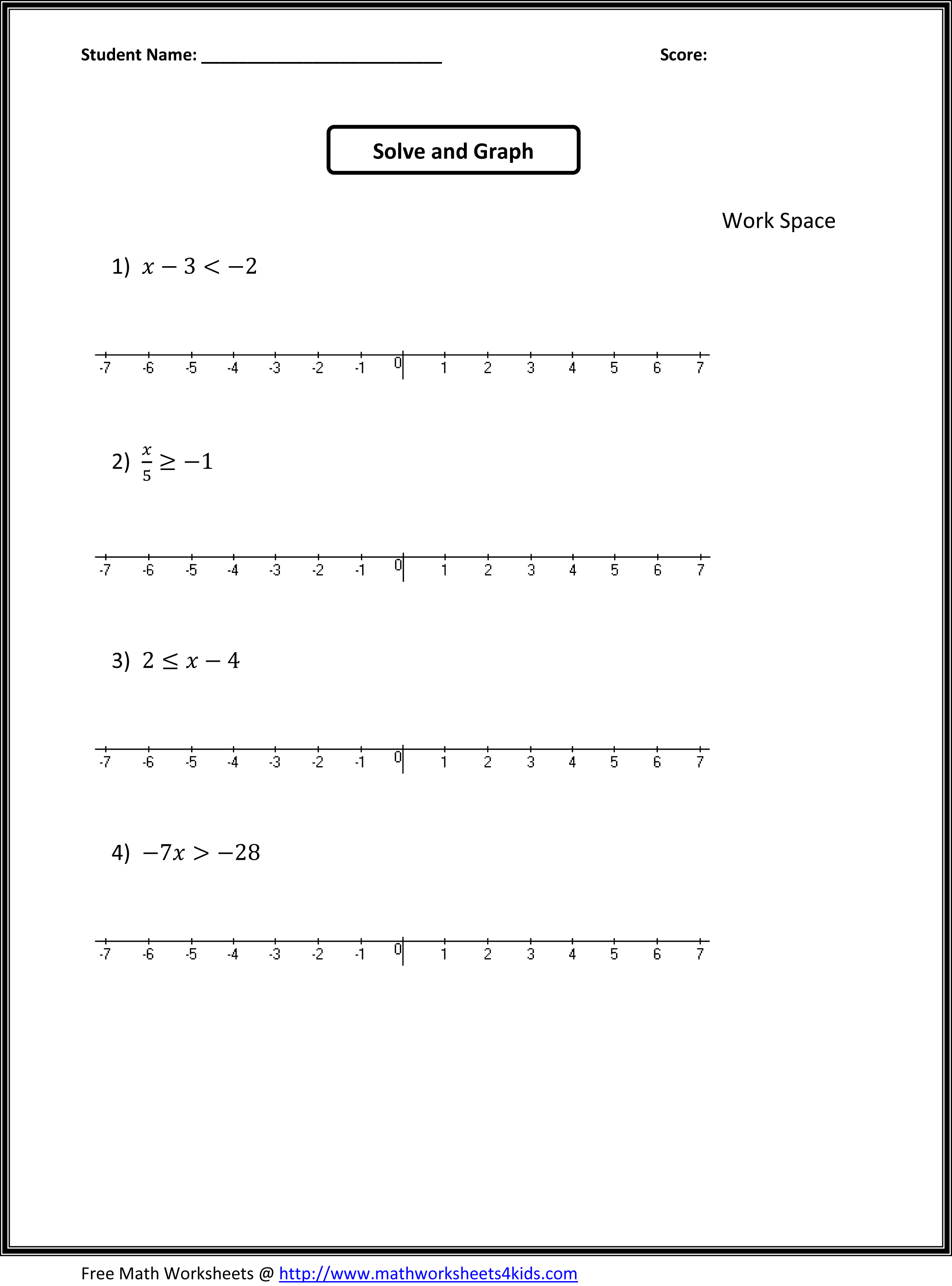
- 7th Grade Math Inequalities Worksheets Printable
- Chemical Word Equations Worksheet Answers
- Chemistry Formula Sheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the purpose of balancing equations in chemistry?
The purpose of balancing equations in chemistry is to ensure that the number of atoms of each element on both sides of the chemical equation is equal. By balancing the equation, it allows us to accurately describe the reactants and products involved in a chemical reaction and follow the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Explain what is meant by a balanced chemical equation.
A balanced chemical equation is one in which the number of atoms of each element is the same on both sides of the equation. This means that the law of conservation of mass is satisfied, showing that the total number of atoms of each element remains constant before and after a chemical reaction. In order to balance a chemical equation, coefficients can be added in front of the chemical formulas to ensure that the same number of atoms are present on both sides of the equation.
How do you determine the coefficients in a balanced chemical equation?
To determine the coefficients in a balanced chemical equation, you need to first write out the molecular formula for each reactant and product involved in the reaction. Then, using the principle of conservation of mass, balance the equation by adjusting the coefficients of the molecules such that the number of atoms of each element is the same on both sides of the equation. This process involves trial and error to achieve a balanced equation where the total mass of the reactants equals the total mass of the products.
What is the law of conservation of mass and how is it related to balancing equations?
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, only rearranged. Balancing chemical equations ensures that this law is obeyed by maintaining the same number of atoms of each element on both sides of the equation. Balancing equations involves adjusting coefficients to ensure that the same number of atoms of each element is present in the reactants and products, allowing for a proper representation of the conservation of mass in chemical reactions.
Describe the steps involved in balancing a chemical equation.
To balance a chemical equation, first write down the unbalanced equation. Then, start by balancing the most complex or compound that appears in only one place. Adjust the coefficients to ensure the same number of atoms for each element on both sides of the equation. Continue balancing each element one at a time while double-checking that all atoms are accounted for. If needed, consider modifying coefficients that were previously balanced. Finally, ensure that the final balanced equation is in the simplest whole-number ratios.
Why is it important to balance both the atoms and the charges in a chemical equation?
Balancing both the atoms and the charges in a chemical equation is important because it ensures that the law of conservation of mass and charge is obeyed. This means that matter is neither created nor destroyed during a chemical reaction, and that the total charge is conserved. Balancing the equation also allows for accurate calculations of reactants and products in a reaction, ensuring that the stoichiometry is correct. Furthermore, a balanced equation provides information on the ratio of reactants and products involved in the reaction, aiding in the prediction of the outcome of a chemical reaction.
What does it mean if a chemical equation is not balanced?
If a chemical equation is not balanced, it means that the number of atoms of each element is not equal on both sides of the equation. This can result in incorrect information about the reaction, as the quantities of substances involved are not accurately represented. Balancing a chemical equation is important to ensure that the law of conservation of mass is obeyed, meaning that the same number and types of atoms are present on both sides of the equation.
Give an example of an unbalanced equation and explain how to balance it.
An example of an unbalanced equation is \(\text{Mg} + \text{HCl} \rightarrow \text{MgCl}_2 + \text{H}_2\). To balance it, follow these steps: Start by balancing the elements that appear in only one reactant and one product. Here, you could balance Mg first by adding a coefficient of 1 in front of MgCl2 on the product side. Next, balance Cl by placing a coefficient of 2 in front of HCl. Lastly, balance hydrogen by adding a coefficient of 2 in front of H2. The balanced equation will then be \(\text{Mg} + 2\text{HCl} \rightarrow \text{MgCl}_2 + \text{H}_2\).
Can you balance a chemical equation by changing only the subscripts of the compounds? Why or why not?
No, you cannot balance a chemical equation by changing only the subscripts of the compounds because altering the subscripts changes the identity of the compounds and the overall chemical reaction. To balance a chemical equation, you need to adjust the coefficients of the compounds to ensure that the number of atoms of each element is equal on both sides of the equation. Changing the subscripts would change the molecular formula of the compounds, resulting in a different chemical reaction altogether.
Explain the concept of stoichiometry and its role in balancing equations.
Stoichiometry is the calculation of quantifiable relationships between reactants and products in a chemical reaction. It plays a crucial role in balancing equations by ensuring that the number of atoms of each element is the same on both sides of the equation. By using stoichiometry, we can determine the precise amount of reactants needed to completely react and form the desired products, thus maintaining the conservation of mass in a chemical reaction. Balancing equations using stoichiometry helps us understand the chemical composition of reactions and predict the outcomes of reactions accurately.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments