Balancing Equations Worksheet Answers
Are you struggling to find the answers to your balancing equations worksheet? Look no further! In this blog post, we will provide you with the solutions and explanations you need to master this topic. Whether you are a student trying to improve your understanding of chemistry or a teacher looking for resources to assist your students, this post will guide you through balancing equations with ease.
Table of Images 👆
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Key
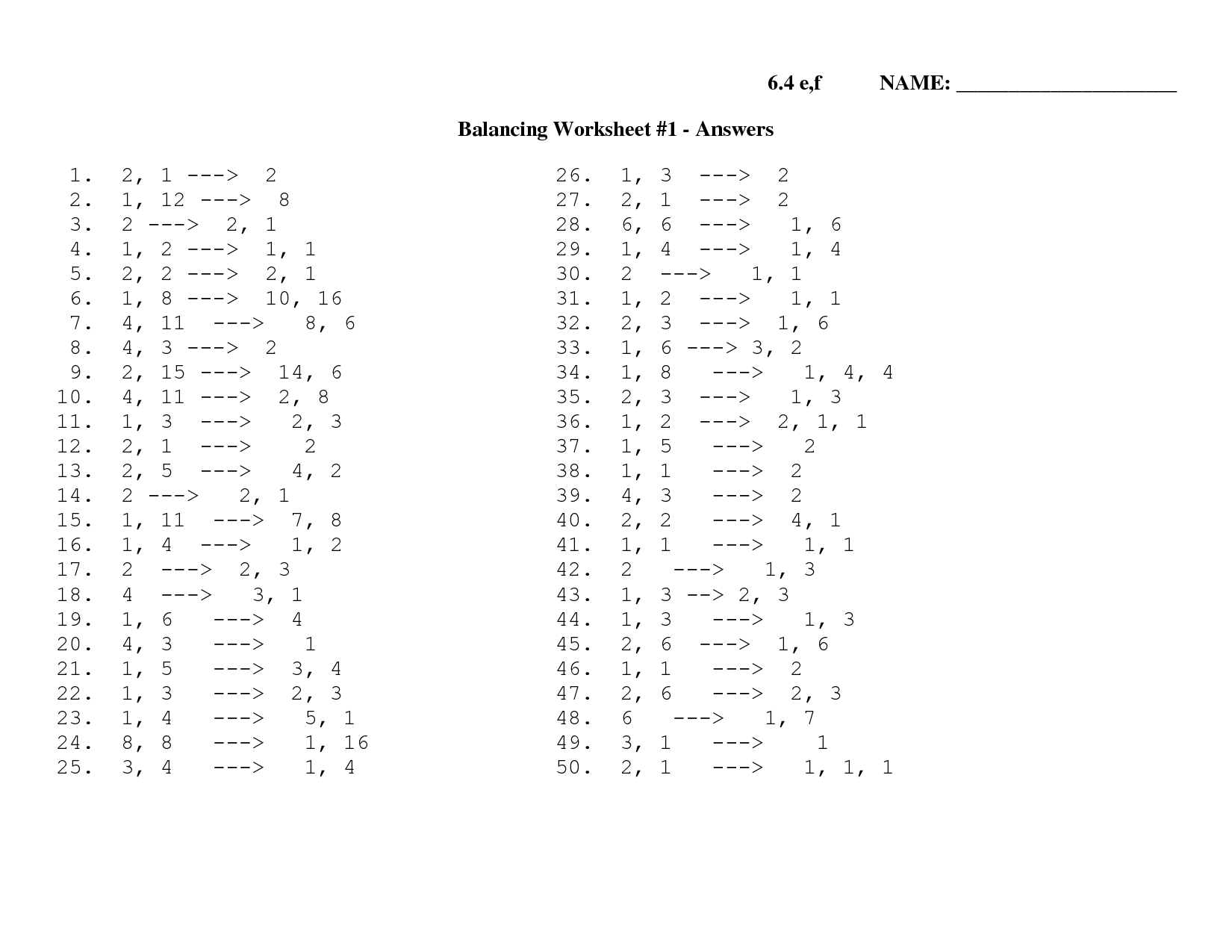
- Balancing Equations Worksheet
- Balancing Equations Practice Worksheet Answers
- Balancing Chemical Equations Worksheet
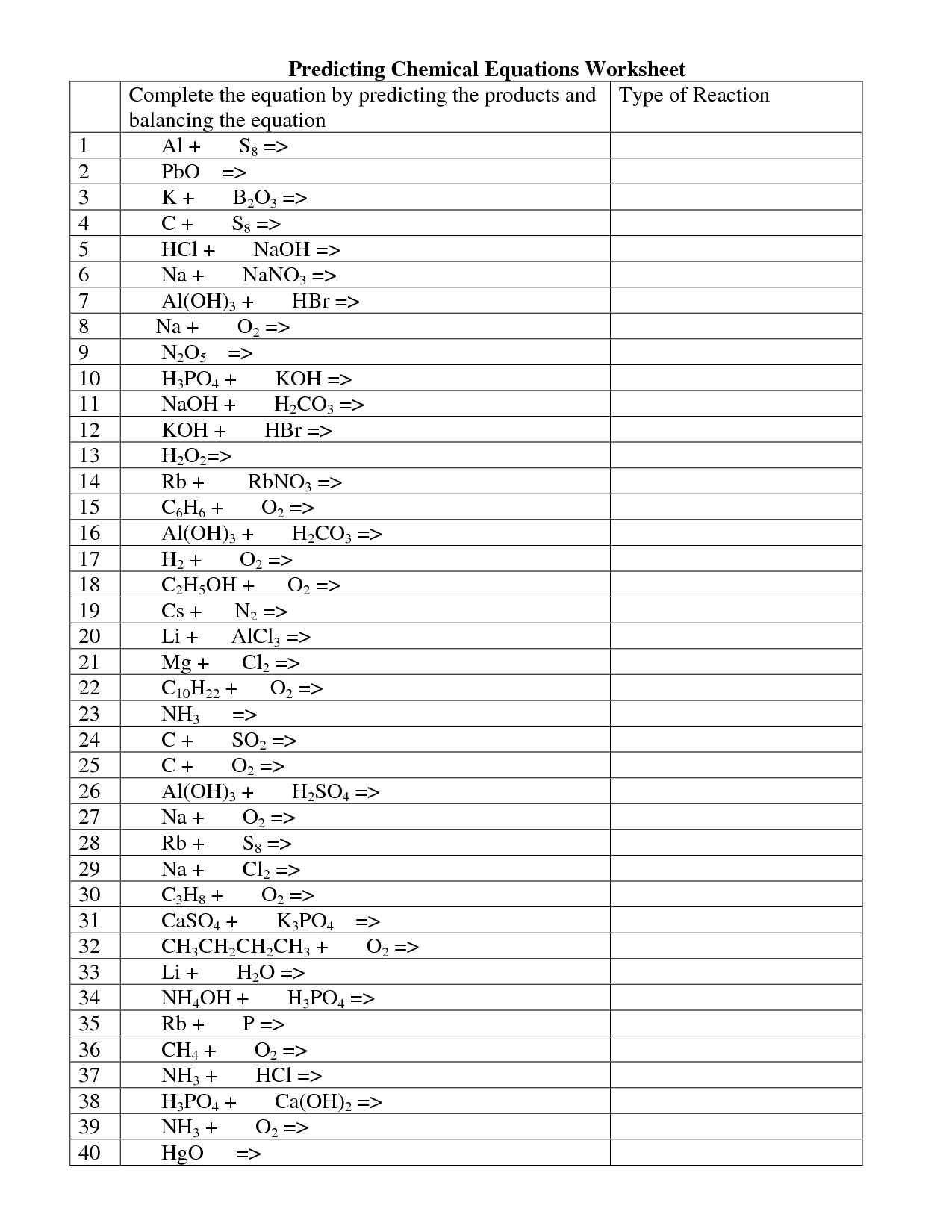
- Predicting Chemical Equations Worksheet
- Chemical Reaction Types Worksheet
- Balancing Chemical Equations Answer Key
- Balancing Chemical Reactions Worksheet
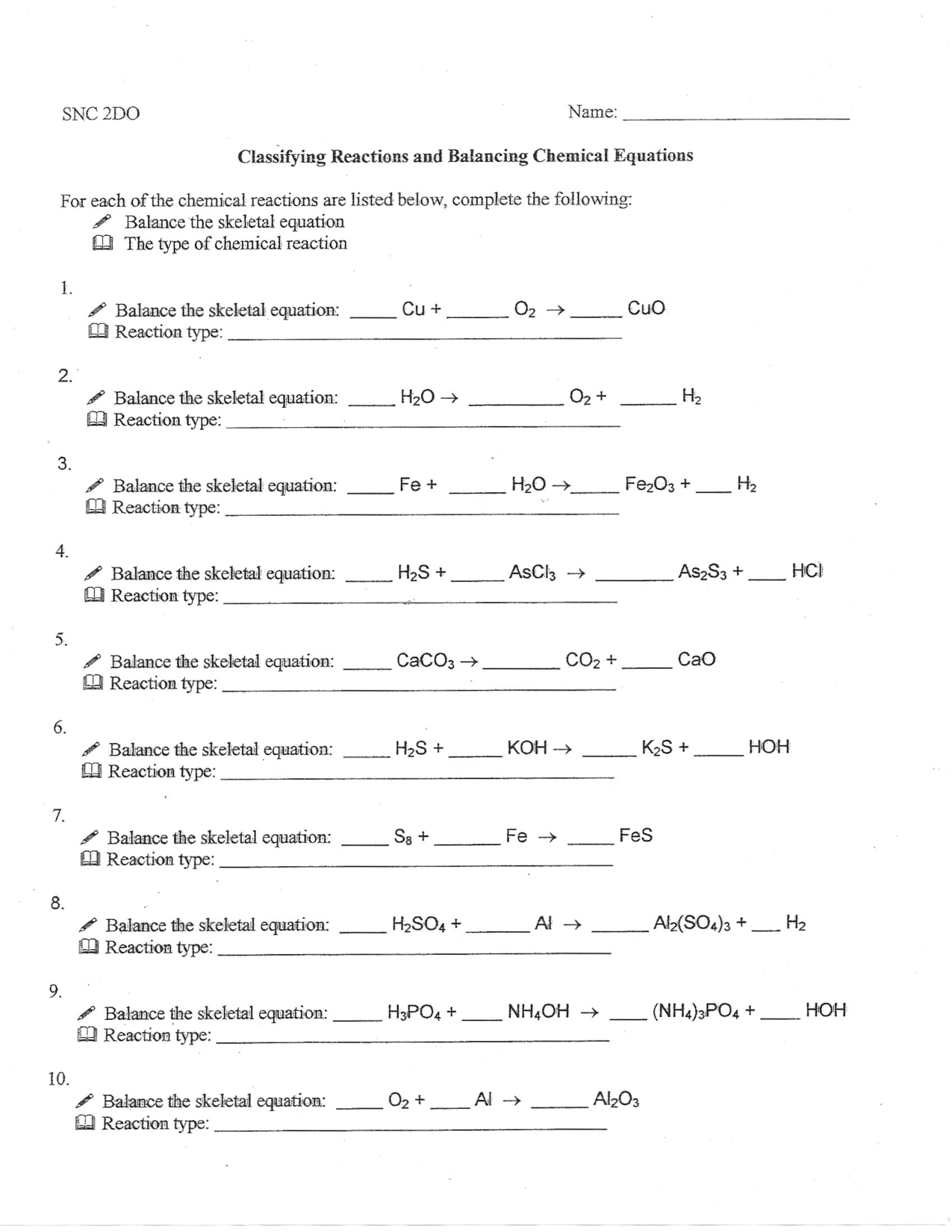
- Classifying Chemical Reactions Worksheet Answers
- Worksheet 11 1 Redox Reactions Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a balanced equation?
A balanced equation is one where the number of atoms of each element on the reactant side is equal to the number of atoms of the same element on the product side. This ensures that the law of conservation of mass is obeyed, meaning that no atoms are lost or gained in a chemical reaction.
How do you determine if an equation is balanced?
To determine if an equation is balanced, you need to ensure that the number of atoms of each element is equal on both sides of the equation. This can be done by counting the number of atoms of each element in the reactants and products and adjusting the coefficients in front of the molecules to balance the equation. Once the equation is balanced, the same number of each type of atom should be present on both the reactant and product sides.
Why is it important to balance chemical equations?
Balancing chemical equations is important because it ensures that the law of conservation of mass is followed. This means that the number of atoms of each element on the reactant side must be equal to the number on the product side. Balancing equations also helps in determining the right stoichiometry of a reaction, showing the correct ratio of reactants and products involved. This is crucial for accurately predicting the outcome of a chemical reaction and for performing experiments in a controlled and reproducible manner.
What are coefficients in a balanced equation?
Coefficients in a balanced equation represent the relative number of moles of each reactant and product involved in a chemical reaction. They are whole numbers placed in front of the chemical formulas to ensure that the equation follows the law of conservation of mass by showing that the same number of atoms of each element are present on both the reactant and product sides of the equation.
How do you balance equations with more complex molecules or polyatomic ions?
To balance equations with more complex molecules or polyatomic ions, it is helpful to first identify all the different elements present on both sides of the equation. Then, adjust the coefficients in front of each compound or element to ensure that the number of atoms of each element is the same on both sides of the equation. It may be necessary to revisit and revise coefficients multiple times to achieve balance. It can also be useful to tackle one element at a time when balancing complex equations. Practice and familiarity with common polyatomic ions and their charges will make balancing equations with these ions easier.
Can you change the subscripts in a chemical formula while balancing equations?
No, you cannot change the subscripts in a chemical formula while balancing equations as the subscripts represent the number of atoms of each element in a compound. When balancing a chemical equation, you must balance it by adding coefficients in front of the chemical formulas to ensure that the number of atoms of each element is the same on both sides of the equation. Changing subscripts would alter the chemical identities of the compounds involved in the reaction.
What are some general tips for balancing equations quickly and efficiently?
Some general tips for balancing equations quickly and efficiently include: 1) Start by balancing atoms that appear only once on each side of the equation, such as elements in compounds or polyatomic ions. 2) Use coefficients to adjust the number of atoms or molecules on each side of the equation. 3) Remember to double-check your work to ensure the equation is balanced. 4) Practice balancing equations regularly to improve your speed and accuracy.
Can you balance equations using fractions as coefficients?
Yes, you can balance equations using fractions as coefficients. To do this, simply treat the fractions like whole numbers during the balancing process. Multiply all coefficients by the denominators to convert the fractions into whole numbers, if needed. Just remember that the goal is to have the same number of each type of atom on both sides of the equation, regardless of whether the coefficients are fractions or whole numbers.
What happens if an equation is not balanced?
If an equation is not balanced, it means that the number of atoms of each element is not equal on both sides of the equation. This can lead to a misunderstanding of the chemical reaction that is taking place, as the reactants are not being fully converted into the correct products. Balancing an equation is essential to accurately represent the chemical reaction and ensure that the law of conservation of mass is obeyed.
How can balancing equations help in predicting the outcome of a chemical reaction?
Balancing equations is crucial in predicting the outcome of a chemical reaction because it ensures that the same number of each type of atom is present on both sides of the equation. By balancing the equation, you can determine the correct stoichiometry of the reaction, which allows you to calculate the amounts of reactants needed and products formed. This helps in understanding the relationship between the reactants and products, predicting the yield of a reaction, and determining the limiting reactant.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments