Balancing Chemical Word Equations Worksheet
Are you a student looking for a useful tool to practice and enhance your understanding of chemical word equations? Look no further as we present to you a comprehensive and well-designed balancing chemical word equations worksheet. This worksheet is specially curated for students who are seeking to strengthen their skills in this topic, providing them with a range of exercises and problems to solve.
Table of Images 👆
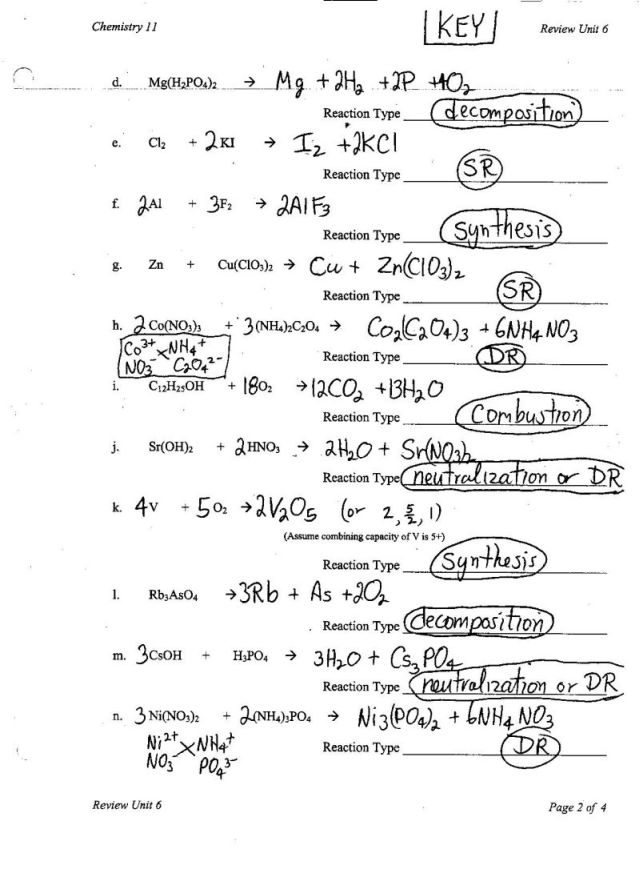
- Balancing Chemical Equations Worksheet 1
- Classifying Chemical Reactions Worksheet Answers
- Balancing Equations Practice Worksheet Answers
- Molarity Stoichiometry Practice Worksheet Answers
- Types of Chemical Reactions Worksheet Answer Key
- Chemical Reaction Types Worksheet
- Mass to Mass Stoichiometry Answer Key
- Sharps Log OSHA Template
- Periodic Table Trends Worksheet Answer Key
More Word Worksheets
Practice Writing Words WorksheetsSpelling Words Worksheets Grade 2
Have Sight Word Worksheet
Fry's First 100 Words Worksheets
First 100 Sight Words Printable Worksheets
Blending Words Worksheets for Kindergarten
9th Grade Worksheets Spelling Words
Matching Definitions to Words Worksheets
Sight Words Worksheets 5th Grade
Element Word Search Worksheet
What is a chemical word equation?
A chemical word equation is a way to represent a chemical reaction using the names of substances involved, rather than chemical formulas. It provides a simple and descriptive way to show the reactants and products of a reaction, making it easier to understand the overall chemical transformation taking place.
How do you determine the balanced equation for a chemical reaction?
To determine the balanced equation for a chemical reaction, you first write down the chemical formulas of the reactants and products involved. Then, you balance the equation by adjusting the coefficients of the compounds so that the number of atoms of each element is equal on both sides of the equation. This ensures that mass is conserved in the reaction, following the law of conservation of mass.
What are the reactants and products in a chemical equation?
Reactants are the substances that are present at the start of a chemical reaction and undergo a change, while products are the substances formed as a result of the reaction. In a chemical equation, reactants are typically written on the left side of the equation, separated by a plus sign, and products are written on the right side, also separated by a plus sign. Symbols and coefficients are used to balance the equation by ensuring that the number of atoms of each element is equal on both sides of the equation.
What is the purpose of balancing a chemical equation?
The purpose of balancing a chemical equation is to ensure that the same number of atoms of each element are present on both sides of the equation, representing a conservation of mass principle. This is important for accurately depicting the reactants and products involved in a chemical reaction and following the law of conservation of mass.
How do you identify the coefficients in a balanced chemical equation?
To identify the coefficients in a balanced chemical equation, you look at the numbers placed in front of each molecule or compound. These coefficients indicate the ratio in which the substances react or are produced in the chemical reaction. The coefficients are determined by balancing the equation, ensuring that the number of atoms of each element is the same on both sides of the equation.
What are some strategies for balancing complex chemical equations?
One strategy for balancing complex chemical equations is to first balance atoms that appear in only one reactant and one product. Then, balance the atoms that appear in multiple reactants or products. Another useful technique is to balance the most complex or largest molecules in the equation first and then work on simpler molecules. Additionally, it can be helpful to keep track of the number of atoms for each element on both sides of the equation and adjust coefficients accordingly. Finally, it is important to double-check the equation after balancing to ensure that all atoms are balanced and that the equation is correctly written.
How does the law of conservation of mass apply to balancing chemical equations?
The law of conservation of mass applies to balancing chemical equations by ensuring that the number of atoms of each element on the reactant side is equal to the number of atoms of the same element on the product side. This means that mass is neither created nor destroyed in a chemical reaction, and the total mass of the reactants must equal the total mass of the products. By balancing chemical equations, we ensure that the total number of atoms of each element is the same on both sides of the reaction, thus following the law of conservation of mass.
What are some common mistakes to avoid when balancing chemical equations?
Some common mistakes to avoid when balancing chemical equations include: forgetting to adjust coefficients for all elements in the reaction, changing subscripts in the chemical formulas, not verifying that the number of atoms is equal on both sides of the equation after balancing, and not double-checking the final balanced equation for accuracy. It is important to pay attention to these details to ensure a correct and balanced chemical equation.
How can you check if a chemical equation is balanced correctly?
To check if a chemical equation is balanced correctly, ensure that the number of each type of atom is the same on both sides of the equation. Count the atoms of each element on the reactant and product sides and adjust the coefficients in front of the compounds as needed to balance the equation. Note that subscripts within compounds cannot be changed as they represent the number of atoms in the molecule.
Why is it important to balance chemical equations accurately?
It is important to balance chemical equations accurately because this ensures that the law of conservation of mass is obeyed. Balancing the equations ensures that the number of atoms of each element on both sides of the equation is equal, reflecting the conservation of matter in a chemical reaction. Failing to balance the equation accurately can lead to incorrect calculations and assumptions about the reaction, potentially hindering scientific understanding and practical applications in fields such as chemistry and biology.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments