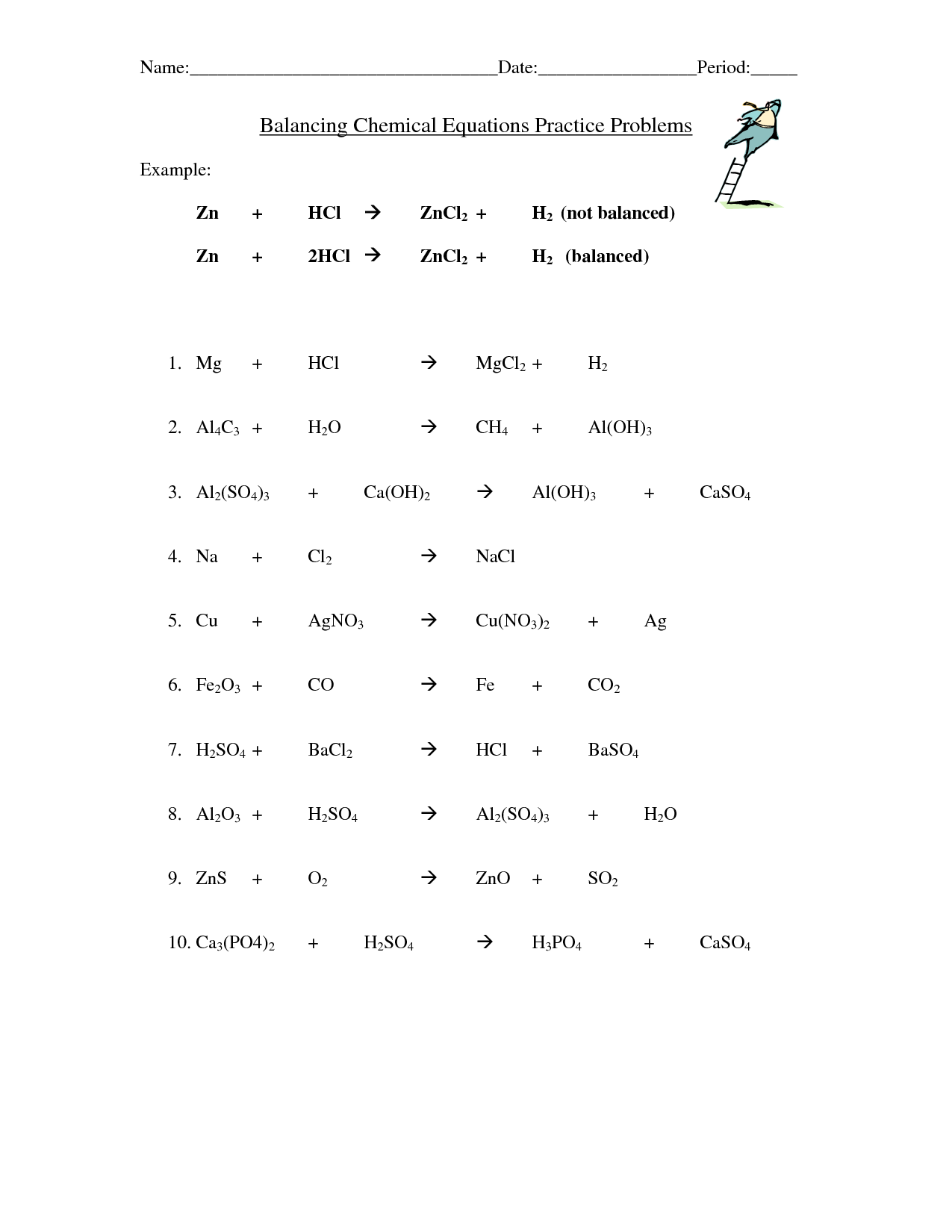
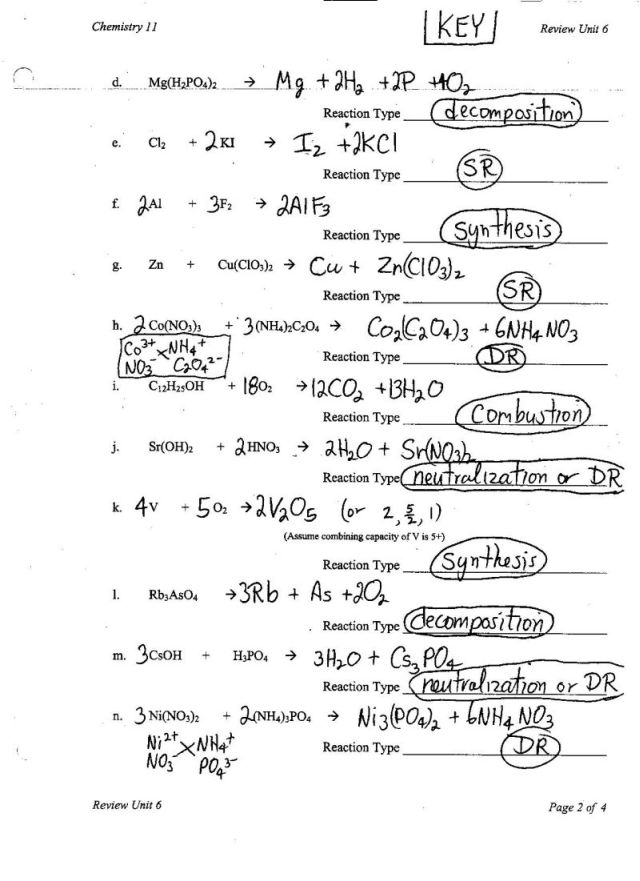
Balancing Chemical Equations Worksheet Easy
Are you struggling to grasp the concept of balancing chemical equations? Look no further! This easy-to-use worksheet will help you understand and master the art of balancing chemical equations. Created specifically for students in introductory chemistry courses, this worksheet provides a comprehensive overview of the topic, making it perfect for beginners who need a solid foundation in this fundamental skill.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a chemical equation?
A chemical equation is a symbolic representation of a chemical reaction using chemical formulas of reactants and products involved in the reaction. It shows the identities and relative amounts of substances before and after the reaction, helping to understand the stoichiometry and balance of the reaction.
How do you balance a chemical equation?
To balance a chemical equation, you need to ensure that the number of atoms of each element is the same on both the reactant and product sides. You can achieve this by adjusting the coefficients in front of each compound in the equation. Start by balancing the more complex or uncommon elements first, then work your way through the equation, making sure to keep the number of atoms balanced on both sides until you have balanced all elements.
What is a coefficient in a chemical equation?
A coefficient in a chemical equation is a number placed in front of a chemical formula to balance the equation by ensuring that the number of atoms of each element is the same on both sides of the equation. It signifies the ratio of molecules or formula units involved in the reaction and helps in maintaining the law of conservation of mass by showing the quantity of each substance taking part in the reaction.
Why is it important to balance chemical equations?
Balancing chemical equations is important because it ensures that the law of conservation of mass is obeyed in a chemical reaction. This means that the total number of atoms of each element is the same on both sides of the equation. Balancing equations provides crucial information about the ratios in which reactants combine and products are formed, allowing researchers to accurately predict and control chemical reactions in various applications such as pharmaceuticals, agriculture, and materials science.
What is the law of conservation of mass and how is it related to balancing chemical equations?
The law of conservation of mass states that mass cannot be created or destroyed in a chemical reaction, only rearranged. When balancing chemical equations, this law must be satisfied by ensuring that the number of atoms of each element is the same on both sides of the equation. This is done by adjusting the coefficients of the compounds in the equation to balance the number of atoms of each element. By balancing the chemical equation, we are upholding the principle of conservation of mass, which is a fundamental concept in chemistry.
Can you balance a chemical equation by changing subscripts? Why or why not?
No, you cannot balance a chemical equation by changing subscripts because altering subscripts changes the identity of the elements involved in the reaction. Balancing a chemical equation requires adjusting coefficients in front of the chemical formulas to ensure that the same number of each type of atom is present on both the reactant and product sides of the equation while keeping the identity of the elements the same.
How do you determine the number of molecules or atoms in a balanced chemical equation?
To determine the number of molecules or atoms in a balanced chemical equation, you need to examine the coefficients that are used to balance the equation. The coefficients represent the ratio of molecules or atoms involved in the reaction. By multiplying the coefficients by the subscripts of the atoms or molecules in the reaction, you can calculate the total number of atoms or molecules involved in the reaction as per the balanced chemical equation.
What are some common techniques used to balance difficult chemical equations?
Some common techniques used to balance difficult chemical equations include starting with the most complex molecules or elements, adjusting coefficients of each compound to ensure that both sides have an equal number of atoms of each element, and using fractions or common multiples to balance any remaining atoms. Additionally, utilizing algebraic principles and trial-and-error methods can help determine the correct coefficients for each compound to achieve a balanced equation.
What are the consequences of not balancing a chemical equation correctly?
Not balancing a chemical equation correctly can lead to incorrect stoichiometry and miscalculation of the quantities of reactants and products involved in a chemical reaction. This can result in failed experiments, wasted materials, and inaccurate conclusions. Balancing a chemical equation is crucial for ensuring that the law of conservation of mass is followed and to accurately predict the outcome of a reaction.
What is the significance of balancing chemical equations in real-life applications?
Balancing chemical equations is essential in real-life applications such as pharmaceuticals, environmental studies, and industrial processes. By ensuring that the number of atoms of each element is the same on both sides of the equation, it helps in accurately determining the quantities of reactants needed, predicting the products formed, and maintaining the efficiency of chemical reactions. This process is crucial for ensuring the safety, effectiveness, and sustainability of various chemical processes in industries like agriculture, food production, and manufacturing.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments