Balancing Chemical Equations Worksheet Answer Key
Chemistry can be a complex subject, especially when it comes to balancing chemical equations. If you're struggling to grasp this fundamental concept, an answer key for balancing chemical equations worksheets can be a valuable resource. This handy tool provides the correct solutions and explanations, helping you better understand the relationship between different elements and their reactions. Whether you're a student trying to improve your grades or a teacher looking for additional teaching materials, an answer key for balancing chemical equations worksheets can be a great asset.
Table of Images 👆
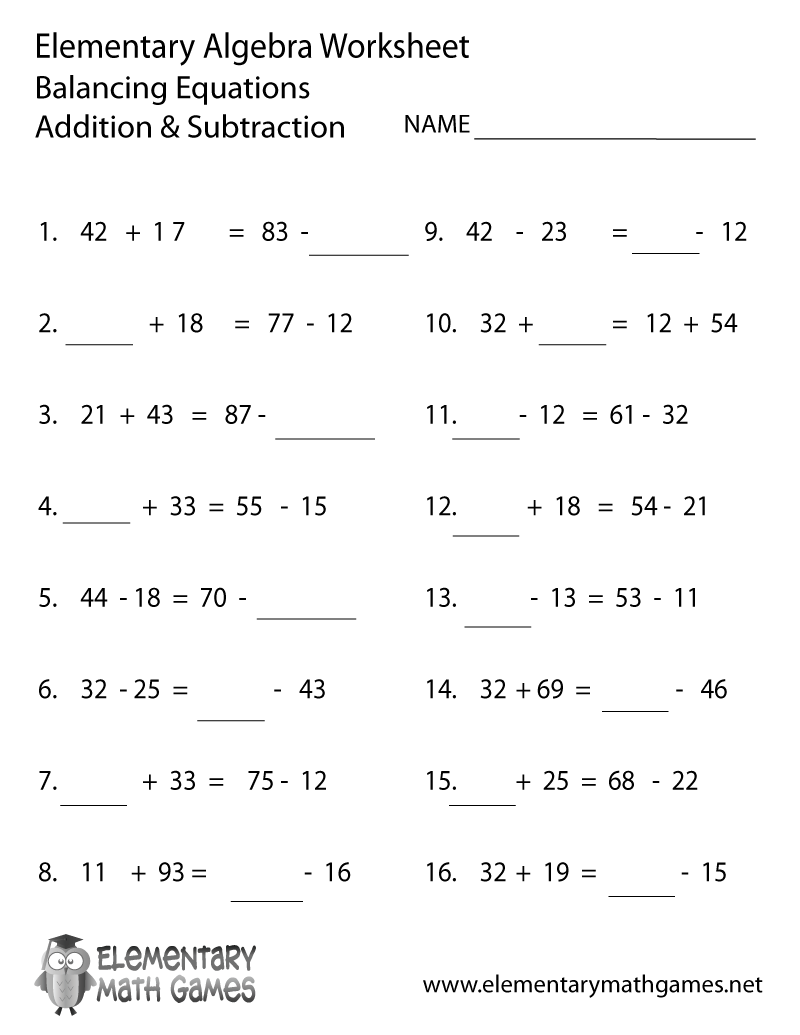
- Free Addition and Subtraction Worksheet
- Balancing Chemical Equations Worksheet Answers
- Balancing Equations Worksheet Answer Key
- Balancing Equations Practice Worksheet Answers
- Chemical Equations Worksheet
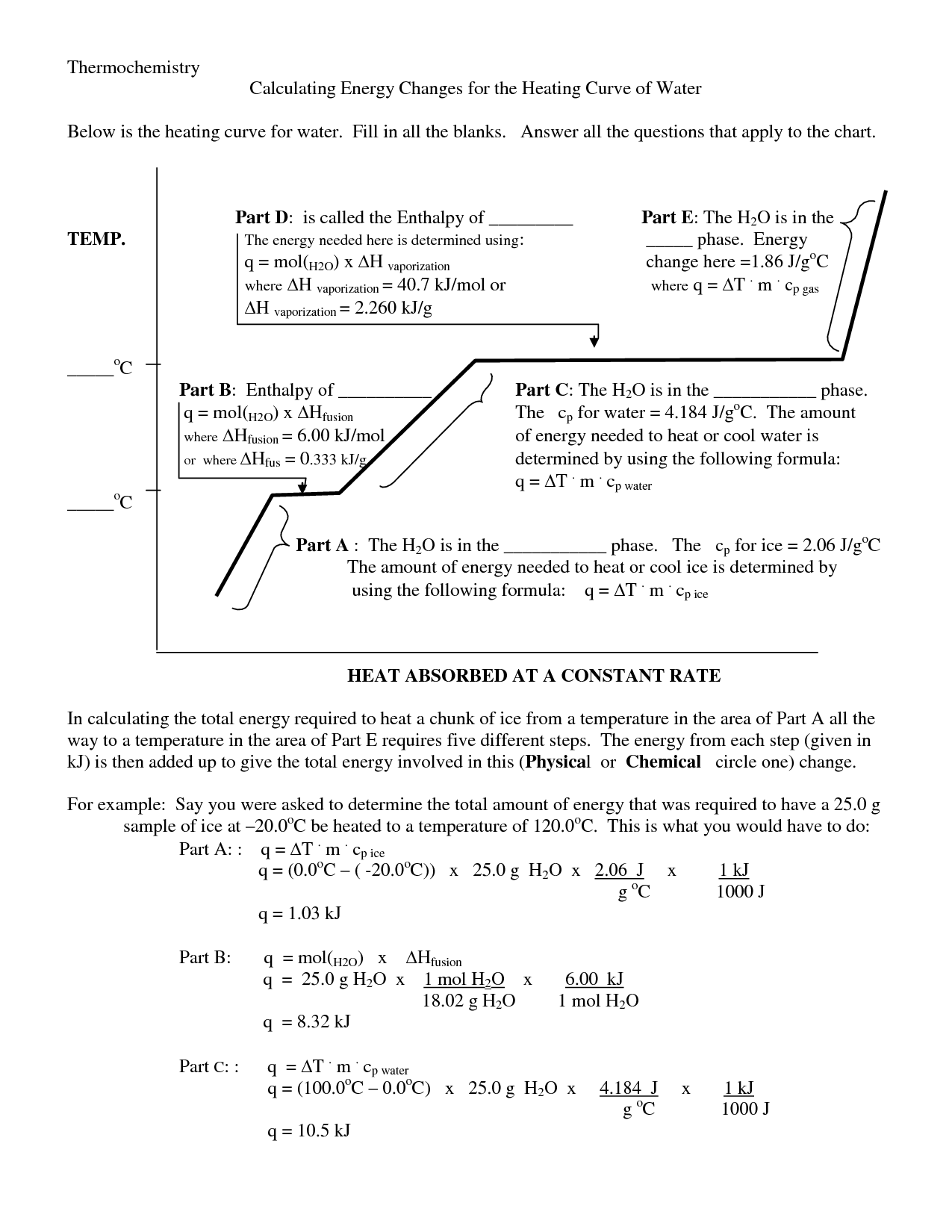
- Heating Curve Kinetic and Potential Energy
- Balancing Equations Worksheet Chemistry If8766
- Decomposition and Synthesis Reactions Worksheet Answers
- Balancing Equations Worksheet 1 Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the purpose of balancing a chemical equation?
The purpose of balancing a chemical equation is to ensure that there is an equal number of atoms of each type on both sides of the equation, representing the law of conservation of mass. Balancing the equation helps to show the chemical reactions accurately by properly representing the reactants and products involved in the reaction, providing information on the stoichiometry and the quantities of reactants and products involved.
How do you know if an equation is balanced?
An equation is considered balanced when the number of atoms of each element in the reactants is equal to the number of atoms of that same element in the products. This is achieved by adjusting the coefficients in front of the chemical formulas to match the number of atoms on both sides of the equation. Illustratively, if you have the equation 2H2 + O2 ? 2H2O, it is balanced because there are 4 hydrogen atoms and 2 oxygen atoms on both sides of the equation.
What are coefficients used for in a balanced chemical equation?
Coefficients in a balanced chemical equation are used to represent the stoichiometry of reactants and products, indicating the relative amounts of each substance involved in a chemical reaction. By adjusting coefficients, one can ensure that the number of atoms of each element is the same on both sides of the equation, maintaining the law of conservation of mass.
What happens if a chemical equation is not balanced?
If a chemical equation is not balanced, it means that the number of atoms of each element is different on both sides of the equation. This can lead to inaccurate predictions of the quantities of substances involved in a reaction. In a balanced equation, the law of conservation of mass is upheld, ensuring that the total number of atoms of each element is the same on both sides. If the equation is not balanced, the reaction may not proceed as expected or the amount of products formed may be incorrect.
How do you balance a chemical equation?
To balance a chemical equation, you need to adjust the coefficients in front of each element or compound in the equation so that the number of atoms of each element is the same on both sides of the equation. Start by balancing the least common elements first and then work your way through the rest of the equation. Remember to only change the coefficients and not the subscripts of the elements. Keep adjusting until the number of atoms of each element is equal on both sides, thus achieving a balanced chemical equation.
Why is it important to have the same number of atoms on both sides of the equation?
It is important to have the same number of atoms on both sides of a chemical equation because of the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Balancing the equation ensures that the same amount of each type of atom is present before and after the reaction, demonstrating that no atoms have been lost or gained during the process.
What are the different methods or strategies for balancing chemical equations?
There are several methods for balancing chemical equations, including the trial and error method, the algebraic method, and the matrix method. The trial and error method involves adjusting coefficients to ensure that the number of atoms of each element is the same on both sides of the equation. The algebraic method involves setting up and solving a system of equations based on the number of atoms of each element. The matrix method involves creating a matrix and using matrix algebra to solve for the coefficients that balance the equation. Each method has its own advantages and may be more suitable for different types of equations.
Can you balance a chemical equation using fractions as coefficients?
No, chemical equations must have whole number coefficients to accurately represent the stoichiometry of a reaction. Fractions are not used as coefficients in balanced chemical equations since they do not represent the physical amounts of reactants and products involved in a reaction.
How do you handle polyatomic ions when balancing chemical equations?
When balancing chemical equations that involve polyatomic ions, treat the polyatomic ion as a single entity. Balance the polyatomic ion as you would do with individual elements or compounds, making sure to keep the entire ion together on both sides of the equation to maintain charge neutrality. Use coefficients to balance the number of each type of polyatomic ion in the reaction.
Why is it important to have an answer key for balancing chemical equations worksheet?
Having an answer key for balancing chemical equations is important because it allows students to self-assess their understanding and verify their work for accuracy. It serves as a tool for immediate feedback, helping students to identify any mistakes they may have made and learn from them. Additionally, it provides a reference point for students to compare their answers, ensuring they are on the right track in mastering this fundamental skill in chemistry.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments