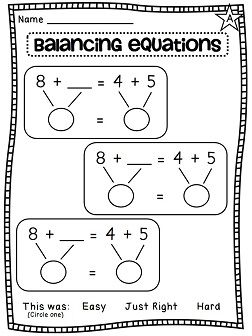
Balance Equations Worksheet Second Grade
Are you a second-grade teacher looking for a fun and engaging way to teach your students about balance equations? Look no further! In this blog post, we will introduce you to a balance equations worksheet specifically designed for second-grade students. This worksheet is a great educational tool that focuses on the concept of balancing equations, helping young learners grasp this mathematical concept in an interactive and hands-on manner.
Table of Images 👆
- 7th Grade Math Algebra Equations Worksheets
- Free Addition and Subtraction Worksheet
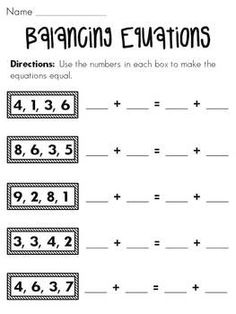
- Balancing Equations Worksheet First Grade Math
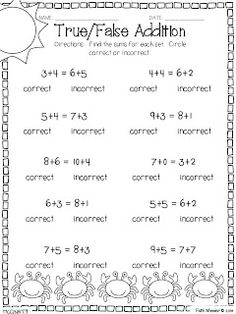
- True False Equations First Grade Worksheet
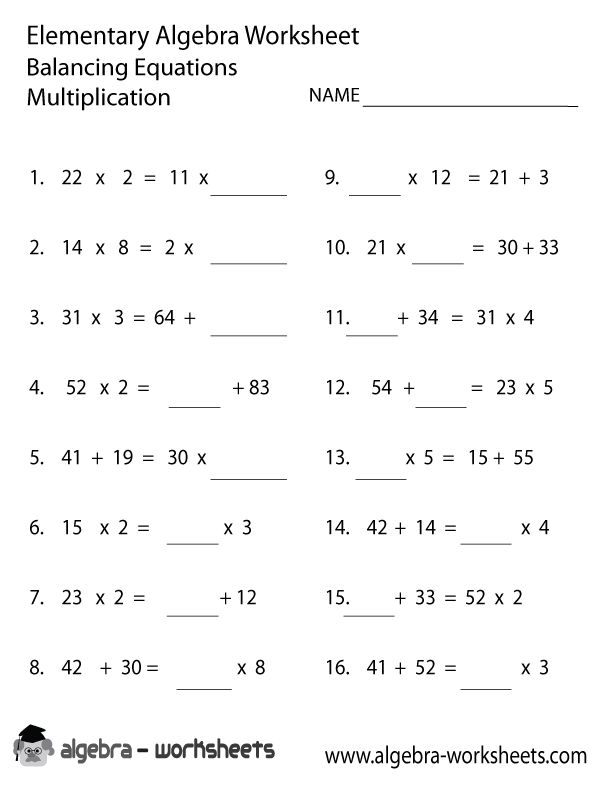
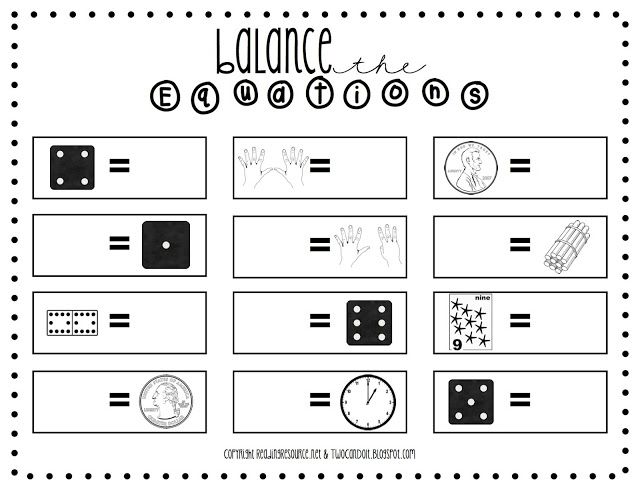
- Balancing Equations Worksheet
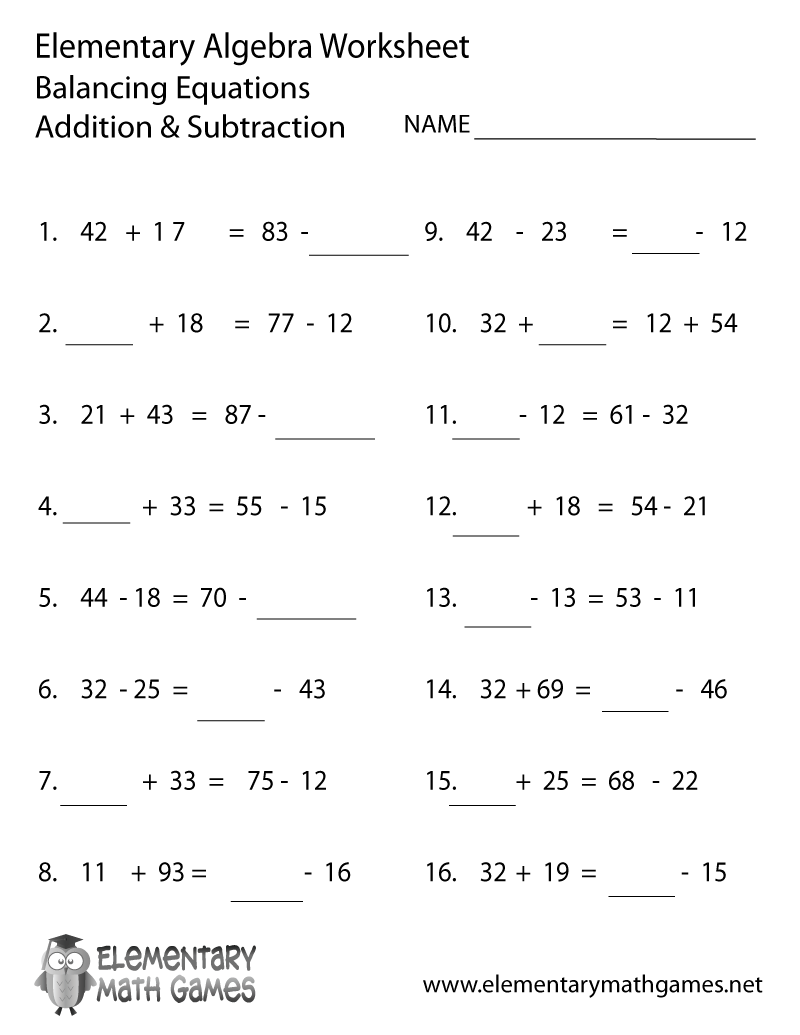
- Balancing Equations Addition Subtraction Worksheets
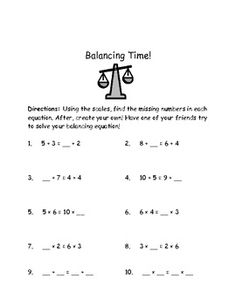
- Balanced Math Equations Worksheets
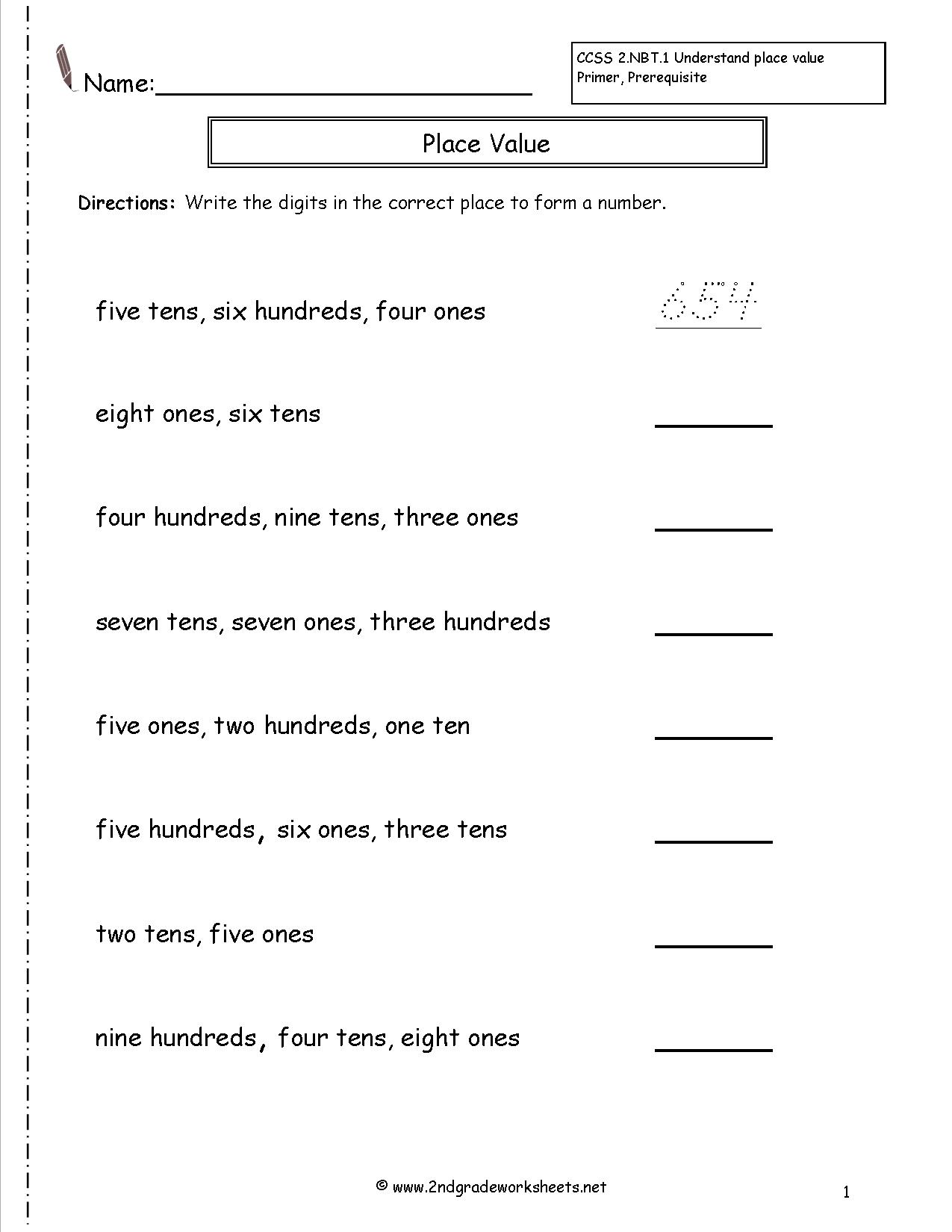
- Place Value Tens and Ones Worksheets
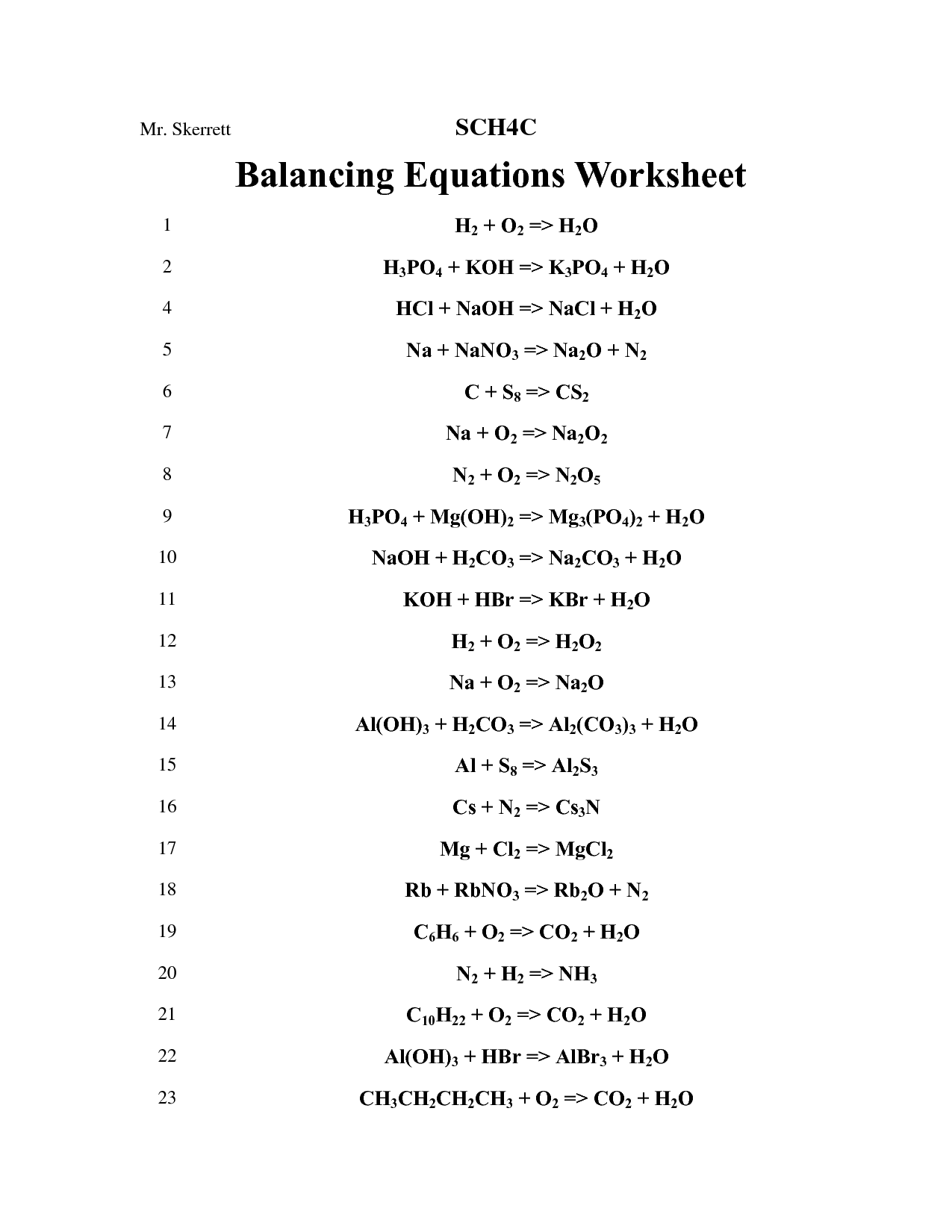
- Balancing Chemical Equations Worksheet Answers
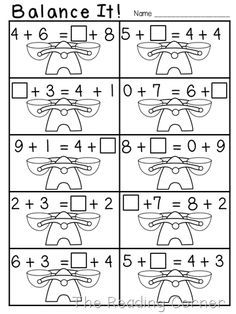
- Balance Equations Worksheets First Grade
- Balancing Chemical Equations Worksheet
- True or False Equations First Grade Worksheet
More 2nd Grade Worksheets
Math Worksheets 2nd Grade ActivitySecond Grade Reading Worksheets Printable
Clock Worksheets for Second Grade
Past Tense Verbs Worksheets 2nd Grade
First Day of School Worksheets 2nd Grade
Main Idea Worksheets Second Grade
Reading Fluency 2nd Grade Worksheets
Second Grade Short Story Worksheet
Being a Good Citizen 2nd Grade Worksheet
What is a balance equation?
A balance equation is a chemical equation that represents a reaction where the number of atoms of each element involved in the reaction is the same on both the reactant and product sides of the equation, ensuring that mass is conserved. It is balanced by adjusting the coefficients of the reactants and products to ensure that the total number of atoms for each element is the same on both sides of the equation.
How can you represent a balance equation with numbers?
To represent a balanced chemical equation with numbers, the coefficients in front of the chemical formulas indicate the ratio in which the reactants combine to form products. For example, in the reaction 2H? + O? -> 2H?O, the numbers 2, 1, and 2 represent the balanced equation where 2 molecules of hydrogen react with 1 molecule of oxygen to form 2 molecules of water. This ensures that the law of conservation of mass is obeyed, showing that the total mass of the reactants equals the total mass of the products.
What are the two sides of a balance equation called?
The two sides of a balance equation are called the reactants and products. The reactants are the starting materials in a chemical reaction, while the products are the substances formed as a result of the reaction. Balancing the equation ensures that there is the same number of atoms of each element on both sides.
How can you keep a balance equation balanced?
To keep a balanced equation balanced, you must make sure that the number of atoms of each element present on the reactant side of the equation is equal to the number of atoms of the same element on the product side. This is achieved by adjusting the coefficients of the compounds in the equation to ensure the conservation of mass, so that the total number of each type of atom is the same on both sides of the equation.
Can you change the order of the numbers in a balance equation?
No, you cannot change the order of numbers in a balanced chemical equation. The coefficients in a balanced equation must represent the correct stoichiometry of the reactants and products, and changing the order of numbers would alter this ratio, leading to an incorrect representation of the chemical reaction.
What happens if you add or subtract numbers on one side of a balance equation?
If you add or subtract numbers on one side of a balanced equation, the equation becomes unbalanced. In order to maintain equality, any changes made to one side of the equation must also be made to the other side. This ensures that the law of conservation of mass is upheld, where the total mass of the reactants must equal the total mass of the products in a chemical reaction.
How can you solve a balance equation if one side is missing?
To solve a balance equation when one side is missing, you can first try to balance the side that is complete. Then, you can systematically manipulate the incomplete side by adding coefficients to each molecule until the number of atoms of each type on both sides are equal. If the equation cannot be balanced by this method, you may need to revisit the provided information or seek additional data to accurately balance the equation.
What does it mean if a balance equation is unbalanced?
If a balance equation is unbalanced, it means that the number of atoms of each element present in the reactants is not the same as that in the products. This can lead to incorrect stoichiometry calculations and inaccurate predictions of reaction outcomes, as the law of conservation of mass is not being upheld. To ensure accuracy in chemical reactions, it is crucial to balance the equation by adjusting the coefficients of the reactants and products until the number of atoms on each side is equal.
What are some real-life examples of balance equations?
Some real-life examples of balance equations include chemical reactions, where the number of atoms of each element must be equal on both sides of the equation, and accounting, where assets must equal liabilities plus equity. Another example is in physics, such as Newton's third law, where the force exerted by one object on another is equal in magnitude and opposite in direction. These balance equations help us understand and predict natural phenomena and maintain equilibrium in various systems.
How can you use balance equations to solve math problems?
Balance equations can be used to solve math problems by setting up an equation with two equal sides representing the quantities on each side of the balance. By manipulating the equation through addition, subtraction, multiplication, or division, you can solve for the unknown variable or value. This method helps in understanding the relationships between different quantities and finding the solution through algebraic manipulation of the equation.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




















Comments