Atoms Family Worksheet
Are you teaching your grade schooler about the different elements in the periodic table? Look no further for engaging and educational resources, as we have created the perfect Atoms Family worksheet just for you! This worksheet is designed to help young learners understand the concept of atoms and their specific properties, making it an ideal tool for parents and teachers seeking to engage their students in a dynamic learning experience.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
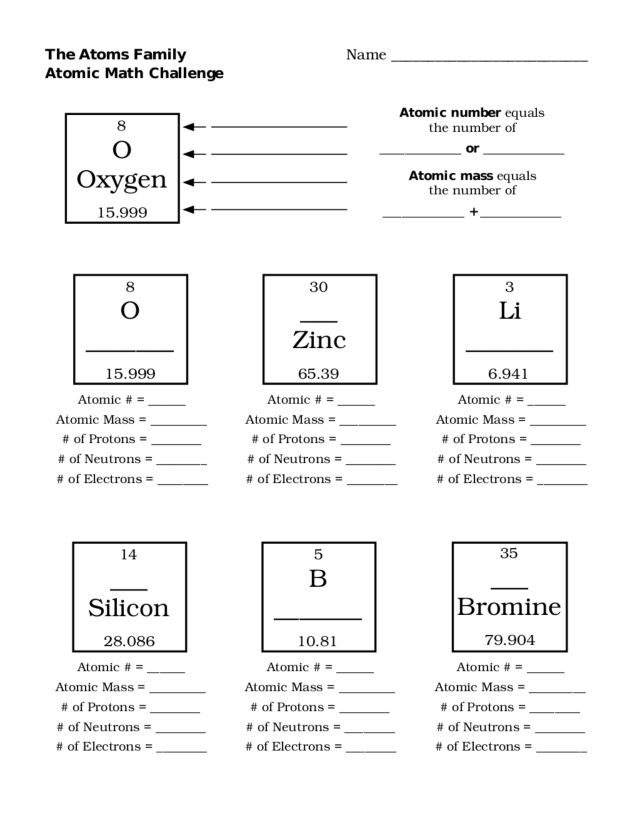
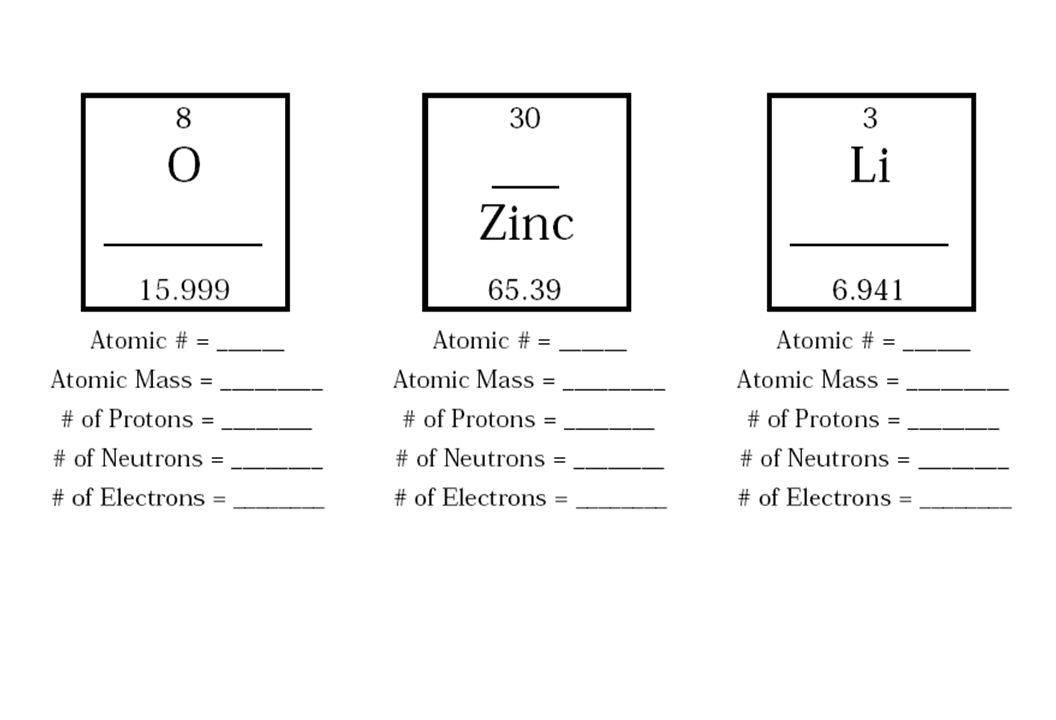
What is an atom?
An atom is the smallest unit of a chemical element that retains the properties of that element. It consists of a nucleus containing protons and neutrons, surrounded by electrons in orbitals. Atoms combine to form molecules through chemical bonds, and they are the building blocks of all matter in the universe.
How are atoms structured?
Atoms are structured with a nucleus at the center, made up of protons and neutrons, surrounded by electrons that orbit the nucleus in energy levels or shells. Protons carry a positive charge, neutrons have no charge, and electrons carry a negative charge. The number of protons in the nucleus determines the element of the atom, while the number of electrons determines its chemical properties and reactivity.
What is the nucleus of an atom and what does it contain?
The nucleus of an atom is the central core that contains protons and neutrons. Protons are positively charged particles, while neutrons have no charge. These two particles make up the majority of an atom's mass. Electrons orbit the nucleus in distinct energy levels but are not located within the nucleus itself.
What are the three primary subatomic particles found in an atom's nucleus?
The three primary subatomic particles found in an atom's nucleus are protons, neutrons, and electrons. Protons are positively charged particles, neutrons are electrically neutral particles, and electrons are negatively charged particles that orbit the nucleus.
What is the charge and mass of a proton?
The charge of a proton is +1 elementary charge, which is approximately 1.602 x 10^-19 coulombs, and the mass of a proton is approximately 1.67 x 10^-27 kilograms.
What is the charge and mass of a neutron?
A neutron has no charge, as it is electrically neutral, and a mass of approximately 1.675 × 10^-27 kilograms, which is slightly greater than that of a proton.
What is the charge and mass of an electron?
The charge of an electron is -1.6 x 10^-19 coulombs, and its mass is approximately 9.11 x 10^-31 kilograms.
Where are electrons located in an atom?
Electrons are located outside the nucleus of an atom in various energy levels or shells.
How do electrons move within an atom?
Electrons move within an atom by circling the nucleus in specific energy levels or orbitals. These orbitals correspond to different energy levels, with electrons occupying the lowest energy levels closest to the nucleus. The movement of electrons is influenced by electromagnetic forces, which dictate their position and behavior within an atom.
What is the overall charge of an atom and why is it neutral?
The overall charge of an atom is neutral because the number of protons in the nucleus, which carry a positive charge, is equal to the number of electrons surrounding the nucleus, which carry a negative charge. This balance of positive and negative charges results in a neutral atom.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments