Atomic Number Worksheet
Are you a chemistry enthusiast, or a student looking to reinforce your understanding of the periodic table? If so, you'll be delighted to know that we have a comprehensive atomic number worksheet that will help you explore the fascinating world of elements and their properties. Our worksheet is designed for individuals who are seeking a hands-on approach to learning about atomic numbers, and it's packed with engaging activities and thought-provoking questions.
Table of Images 👆
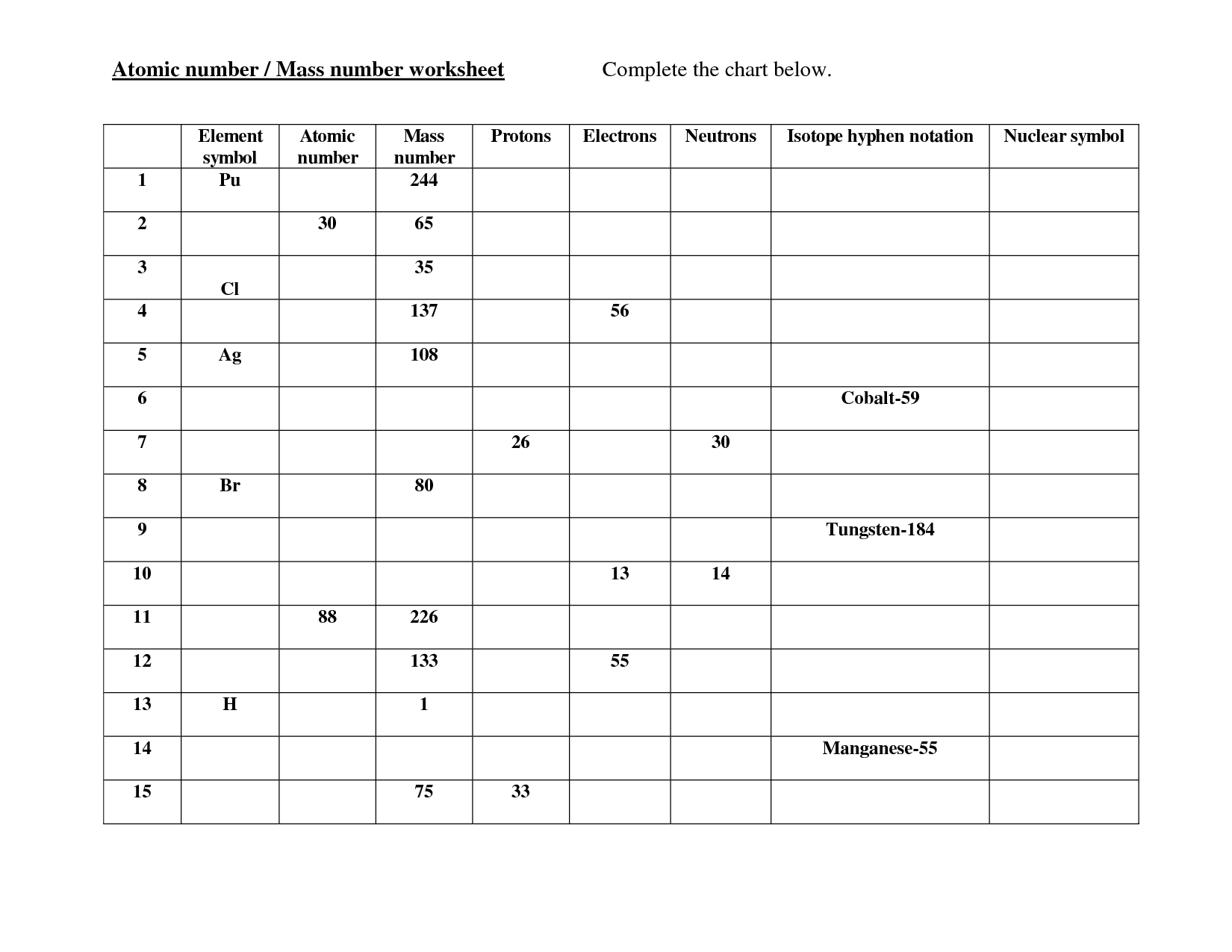
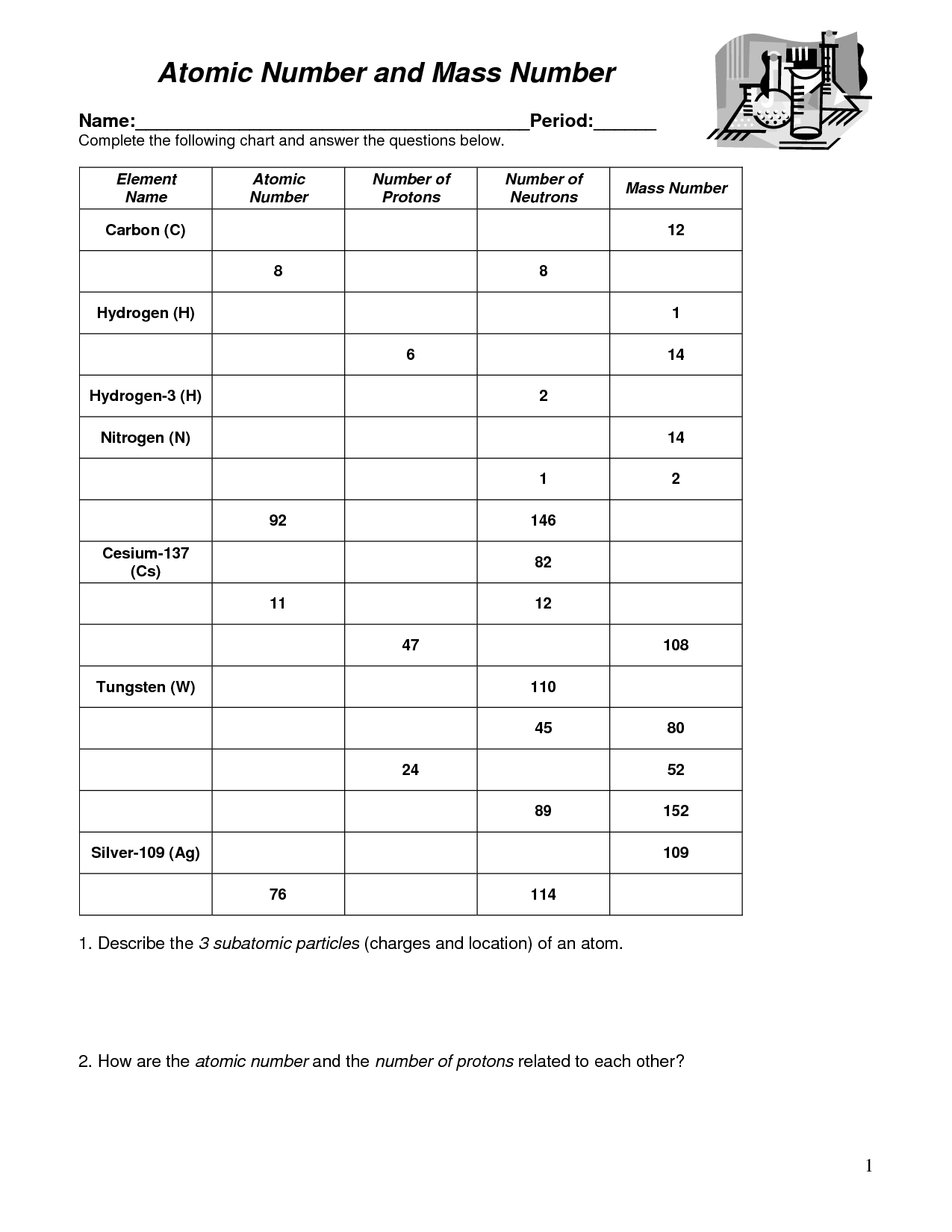
- Mass and Atomic Number Worksheet
- Periodic Table Atomic Number Worksheet
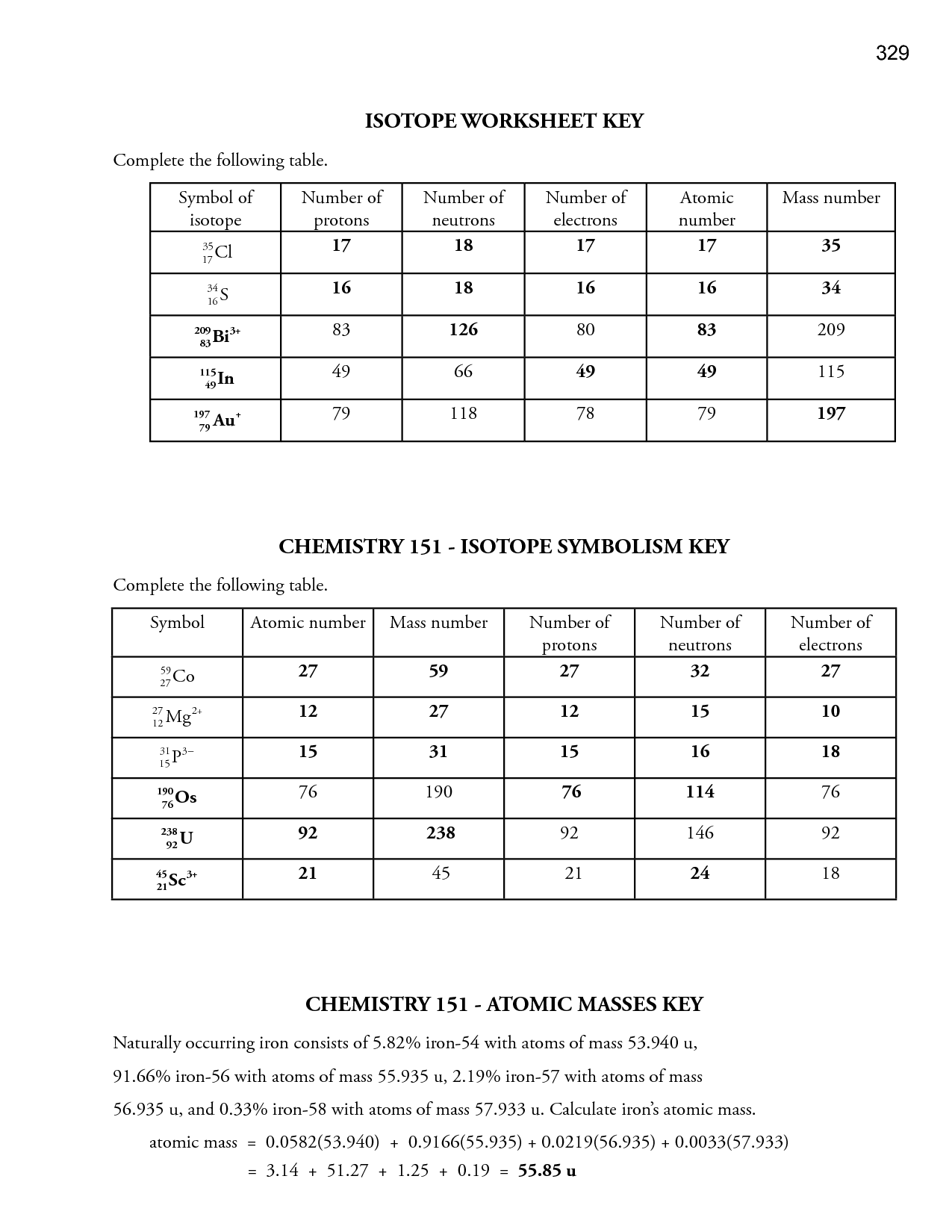
- Isotopes Worksheet Answer Key
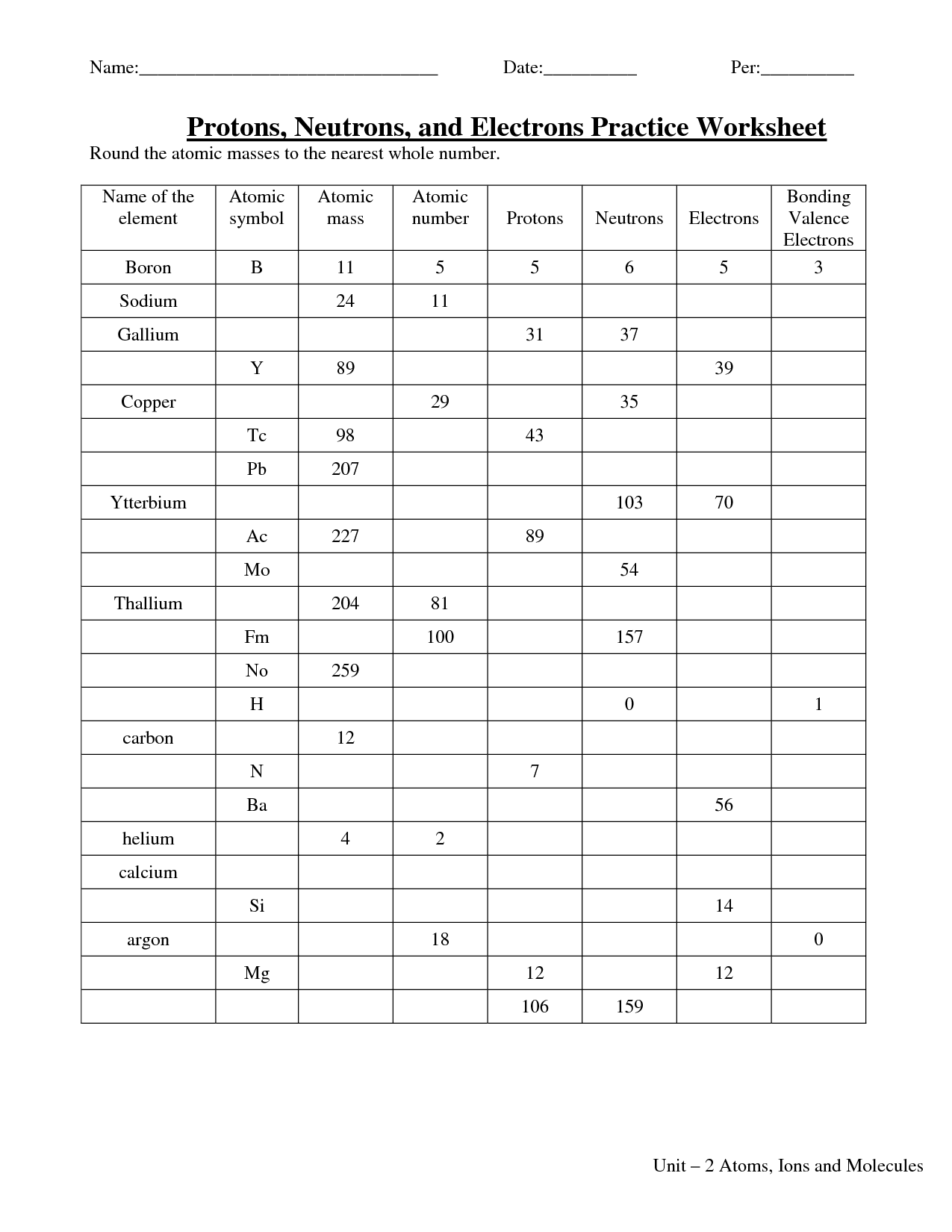
- Protons Electrons Atomic Number Worksheet
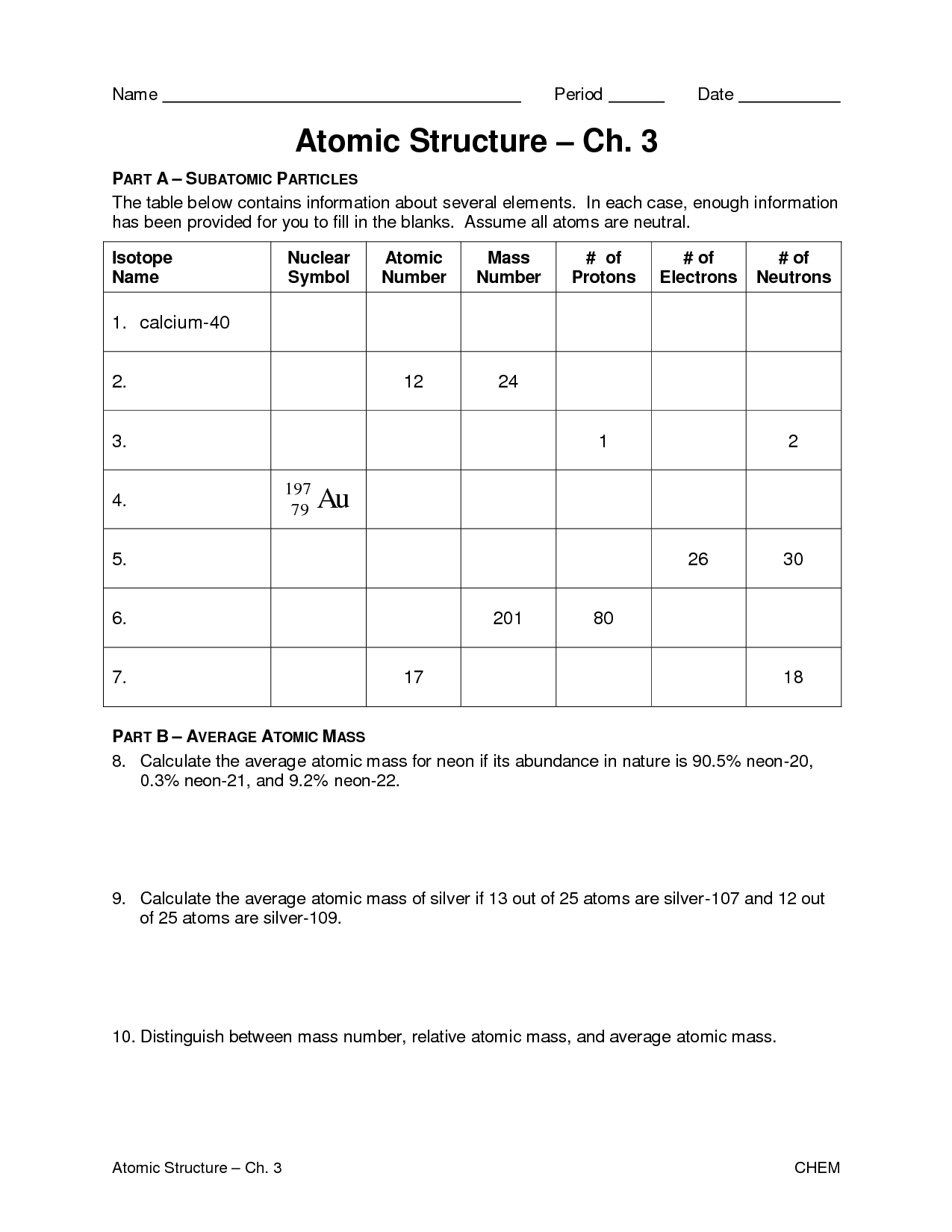
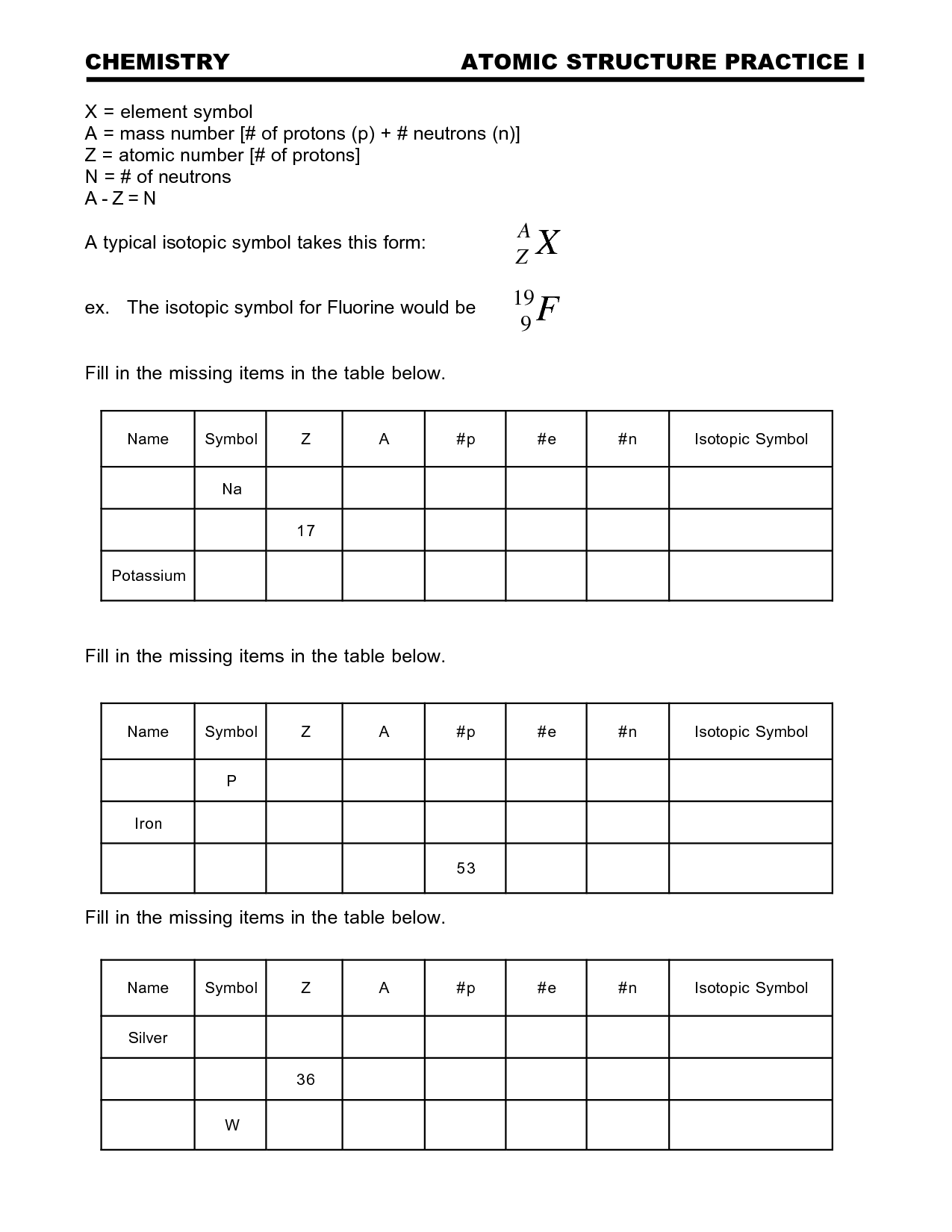
- Atomic Structure Practice Worksheet
- Atomic Mass and Atomic Number Worksheet
- Isotopes Average Atomic Mass and Answers
- Atomic Structure Worksheet Answers
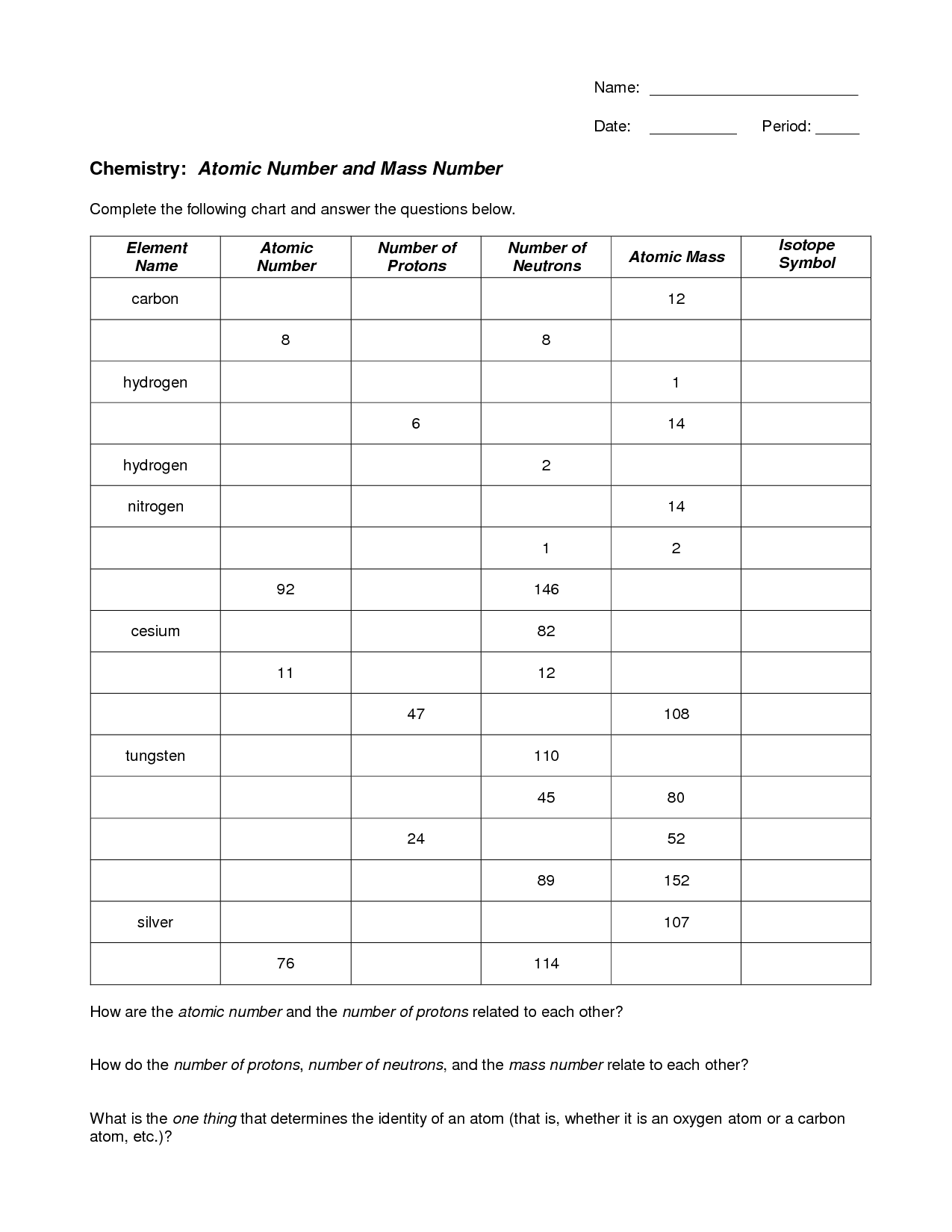
- Atomic Number Protons Neutrons Electrons
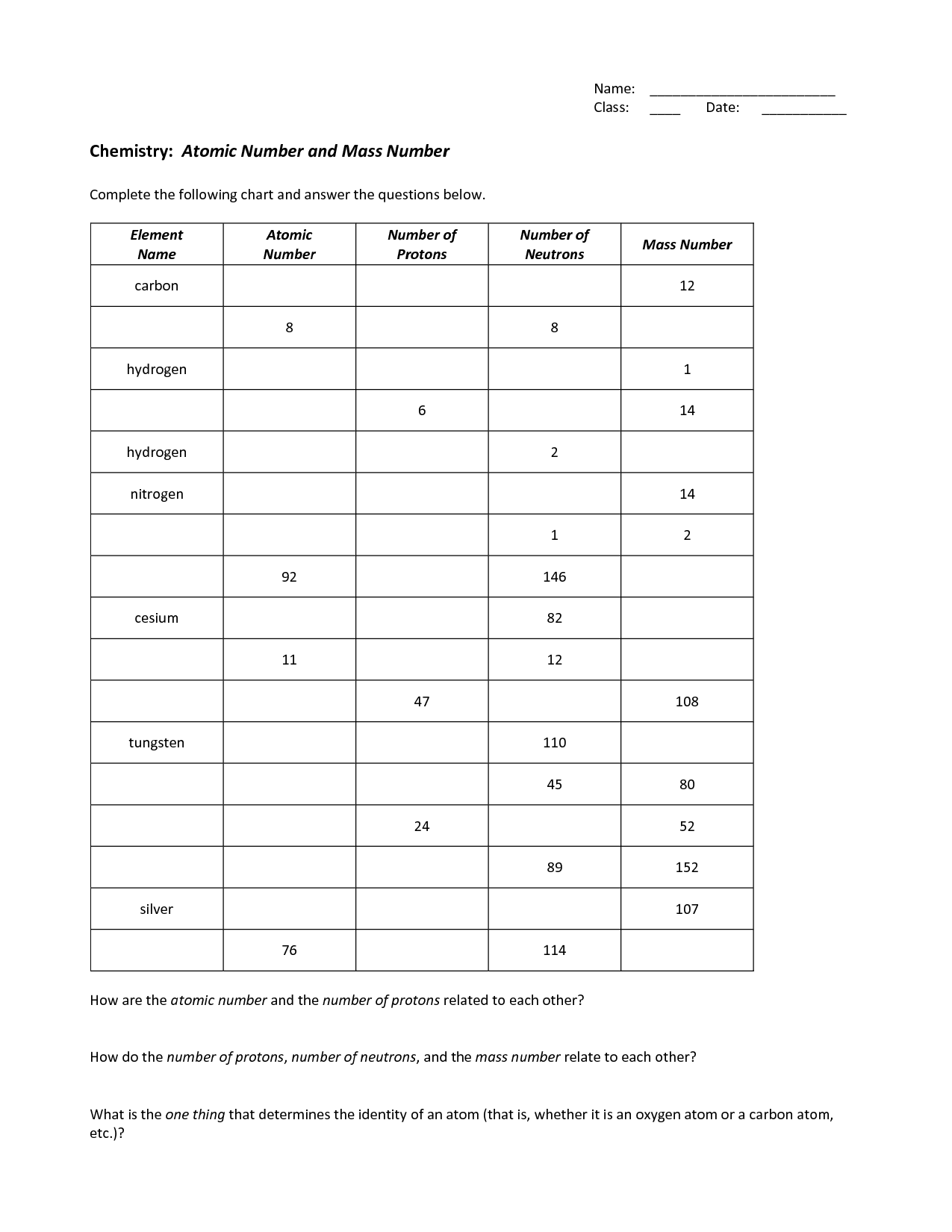
- Basic Atomic Structure Worksheet Chart Answers
More Number Worksheets
Teen Number Practice WorksheetNumber Cut Out Worksheet
Kindergarten Number Worksheets 1 50
Thanksgiving Number Worksheets
Blank Kindergarten Numbers 1-100 Worksheets
Missing Number Multiplication Worksheets
Missing Teen Numbers Worksheet
6th Grade Color by Number Worksheets
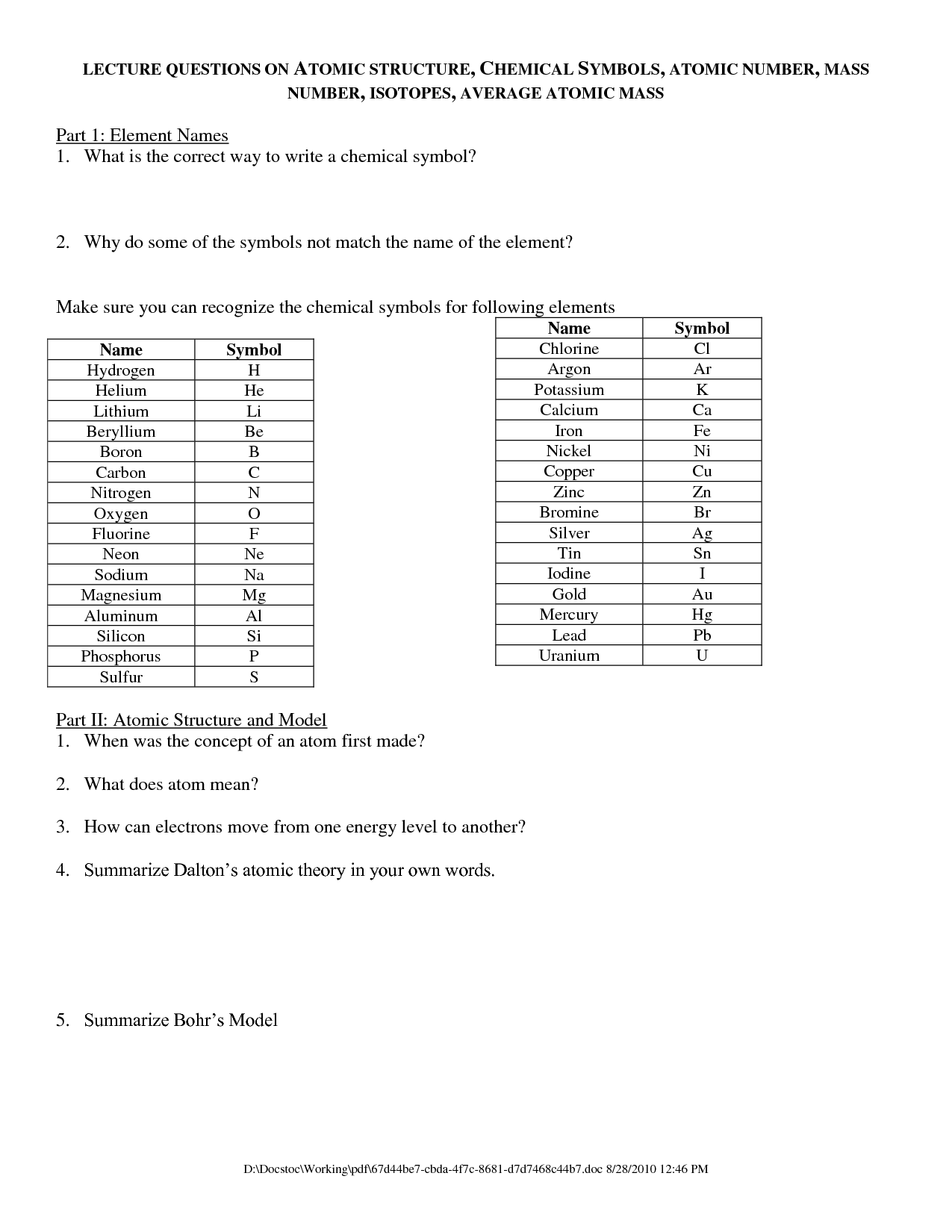
What does the atomic number represent?
The atomic number represents the number of protons in the nucleus of an atom, which determines the element's identity. It also indirectly determines the number of electrons in a neutral atom.
How does the atomic number differ from the mass number?
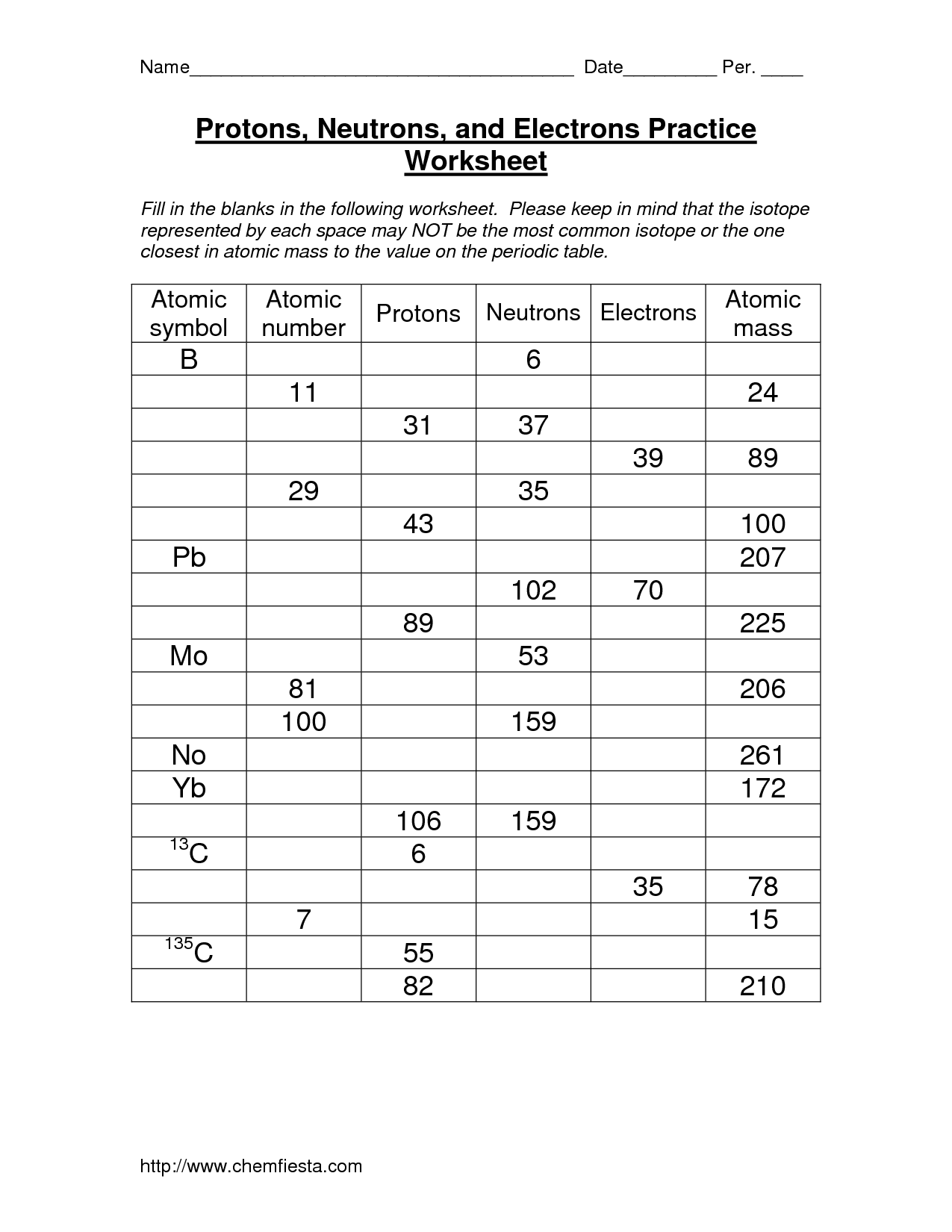
The atomic number is the number of protons in the nucleus of an atom, determining the element's identity, while the mass number is the total number of protons and neutrons in the nucleus. The atomic number identifies the element on the periodic table, whereas the mass number helps distinguish between different isotopes of the same element.
How is the atomic number determined for an element?
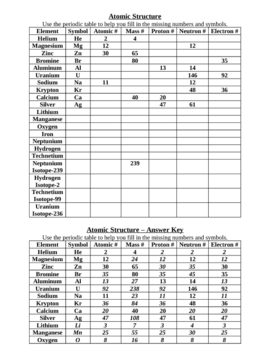
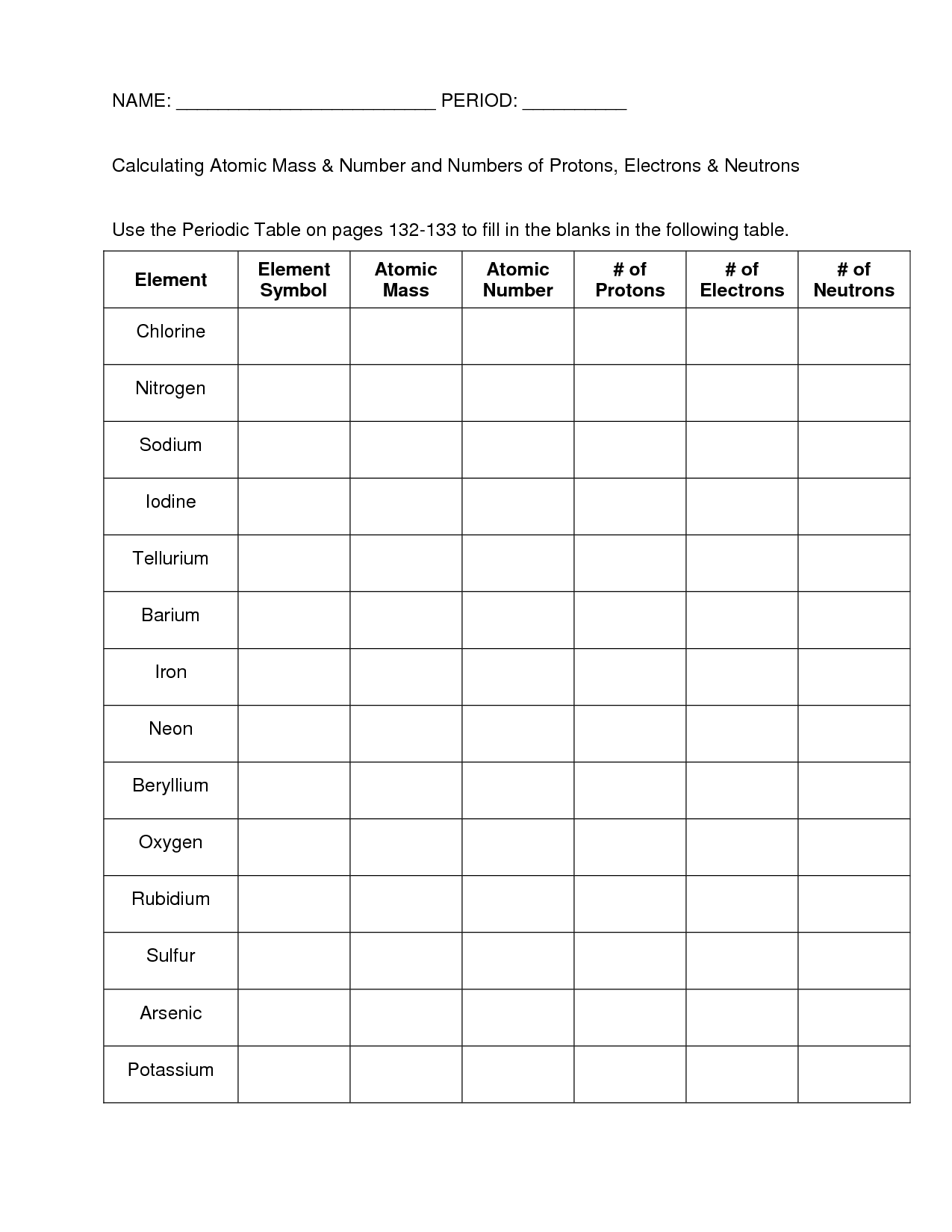
The atomic number of an element is determined by the number of protons in the nucleus of an atom. Each element has a unique atomic number, which defines its place on the periodic table. The atomic number is a fundamental property of an element and can be easily identified based on the number of protons it possesses.
Why is the atomic number important for understanding an element's properties?
The atomic number is important for understanding an element's properties because it determines the element's identity and its placement on the periodic table. The number of protons in an atom's nucleus, which is the same as the atomic number, dictates the element's chemical behavior and physical properties. Elements with the same atomic number share similar characteristics, forming groups and periods on the periodic table that help predict their reactivity, valence electron configuration, and other key properties. In essence, the atomic number is a fundamental characteristic that serves as the foundation for understanding and categorizing elements based on their unique properties.
How does the atomic number relate to the number of protons in an atom?
The atomic number of an atom is equal to the number of protons it contains. Protons are positively charged subatomic particles found in the nucleus of an atom. Therefore, the atomic number serves as a unique identifier for an element and determines its chemical properties.
Can two different elements have the same atomic number?
No, two different elements cannot have the same atomic number. The atomic number of an element is unique to that element and represents the number of protons in the nucleus of its atoms. Different elements have different numbers of protons in their nuclei, which is why they have distinct atomic numbers.
How does the atomic number affect an element's position on the periodic table?
The atomic number of an element determines its position on the periodic table. The periodic table is arranged in order of increasing atomic number, with elements in the same column having similar chemical properties. The atomic number also indicates the number of protons in an atom's nucleus, which determines its unique identity and place on the periodic table.
What is the relationship between the atomic number and the number of electrons in an atom?
The atomic number of an atom is equal to the number of protons in its nucleus, which also corresponds to the number of electrons in a neutral atom. This relationship is crucial as atoms are electrically neutral, and the number of positive protons in the nucleus must be balanced by an equal number of negative electrons orbiting the nucleus to maintain overall electrical neutrality.
How does the atomic number determine the chemical behavior of an element?
The atomic number of an element determines the number of protons in its nucleus, which in turn determines its unique chemical identity. The number of protons also influences the arrangement of electrons in the atom, which plays a significant role in determining the element's chemical behavior. Specifically, the number and distribution of electrons affect how an element will bond with other elements and participate in chemical reactions, ultimately defining its characteristic properties and behavior in chemical reactions.
Can the atomic number of an element change?
No, the atomic number of an element cannot change. The atomic number is a unique identifier for each element and represents the number of protons in an atom's nucleus. Since the number of protons determines the identity of an element, it remains constant for a particular element.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



















Comments