Atomic Model Worksheet
Are you a science teacher searching for a valuable resource to help your students understand the atomic model? Look no further! Our atomic model worksheet provides an engaging and informative activity for students to explore the fundamental building blocks of matter.
Table of Images 👆
- Periodic Table Elements and Atoms Worksheets
- Bohr Model of the Atom Worksheet Answers
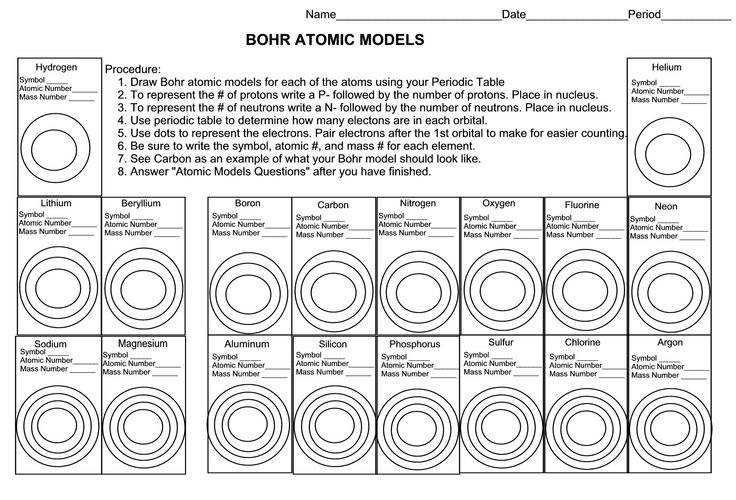
- Bohr Atomic Models Worksheet Answers
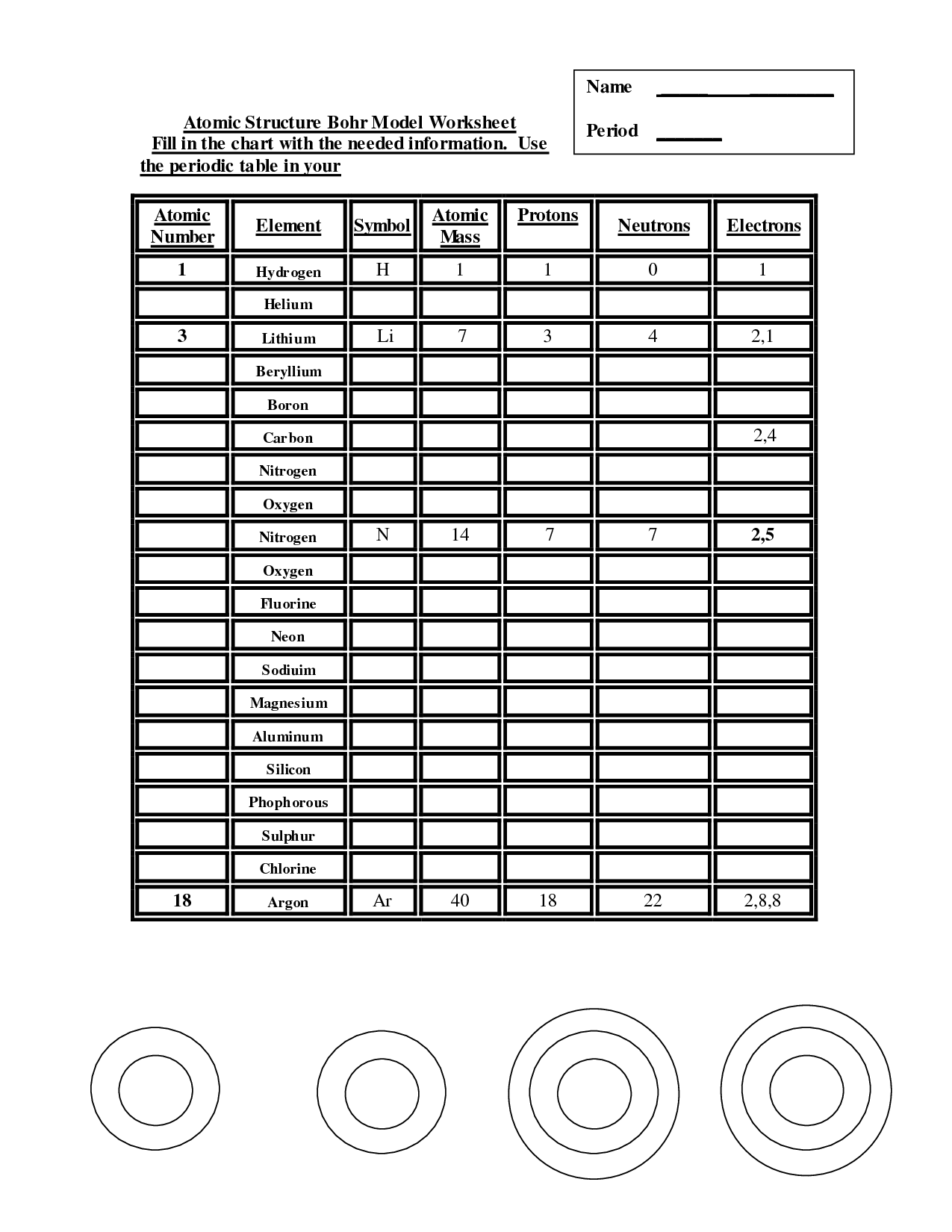
- Atomic Structure Bohr Model Worksheet
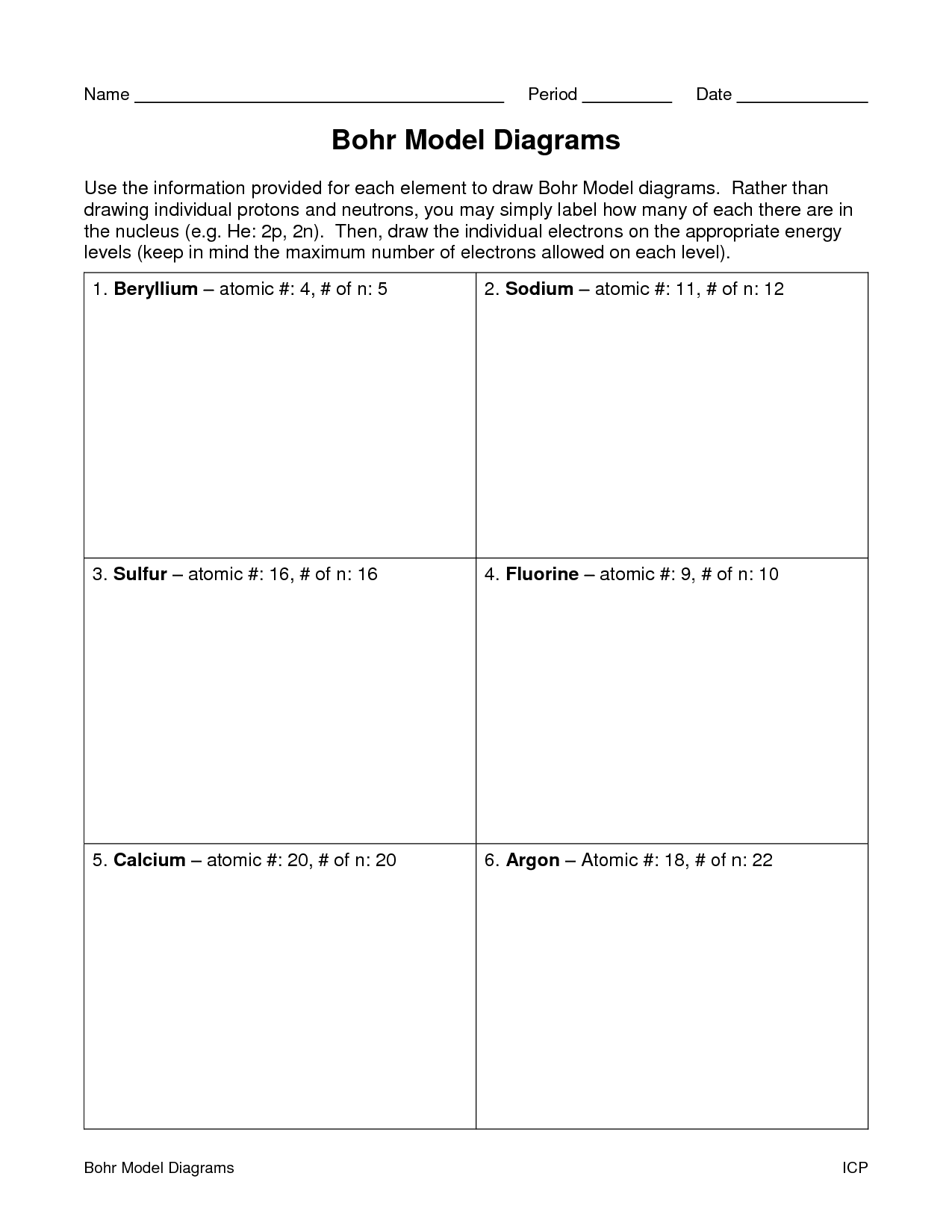
- Blank Bohr Model Worksheet
- The Periodic Table Elements and Atoms Worksheet Answers
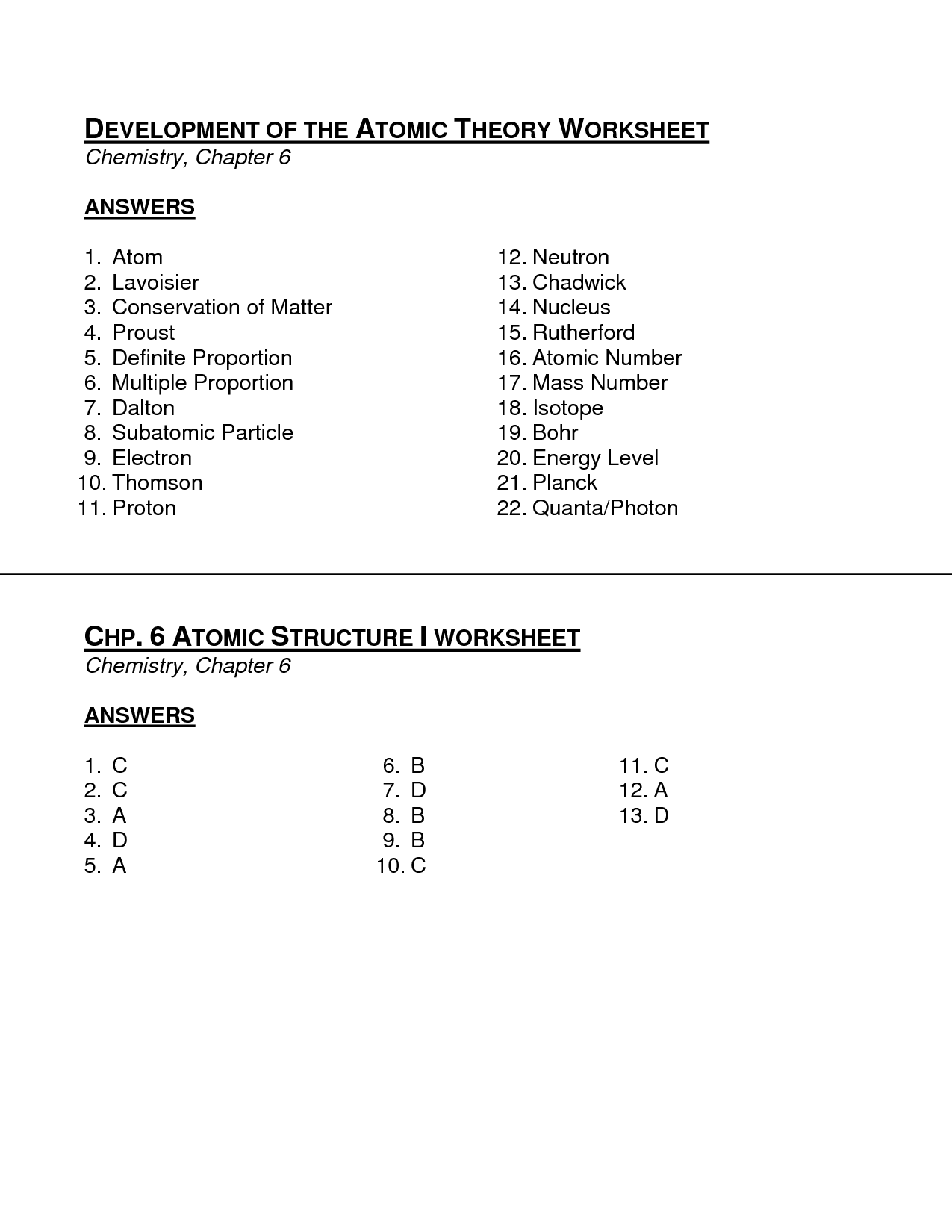
- Theory and Atomic Structure Worksheet Answers
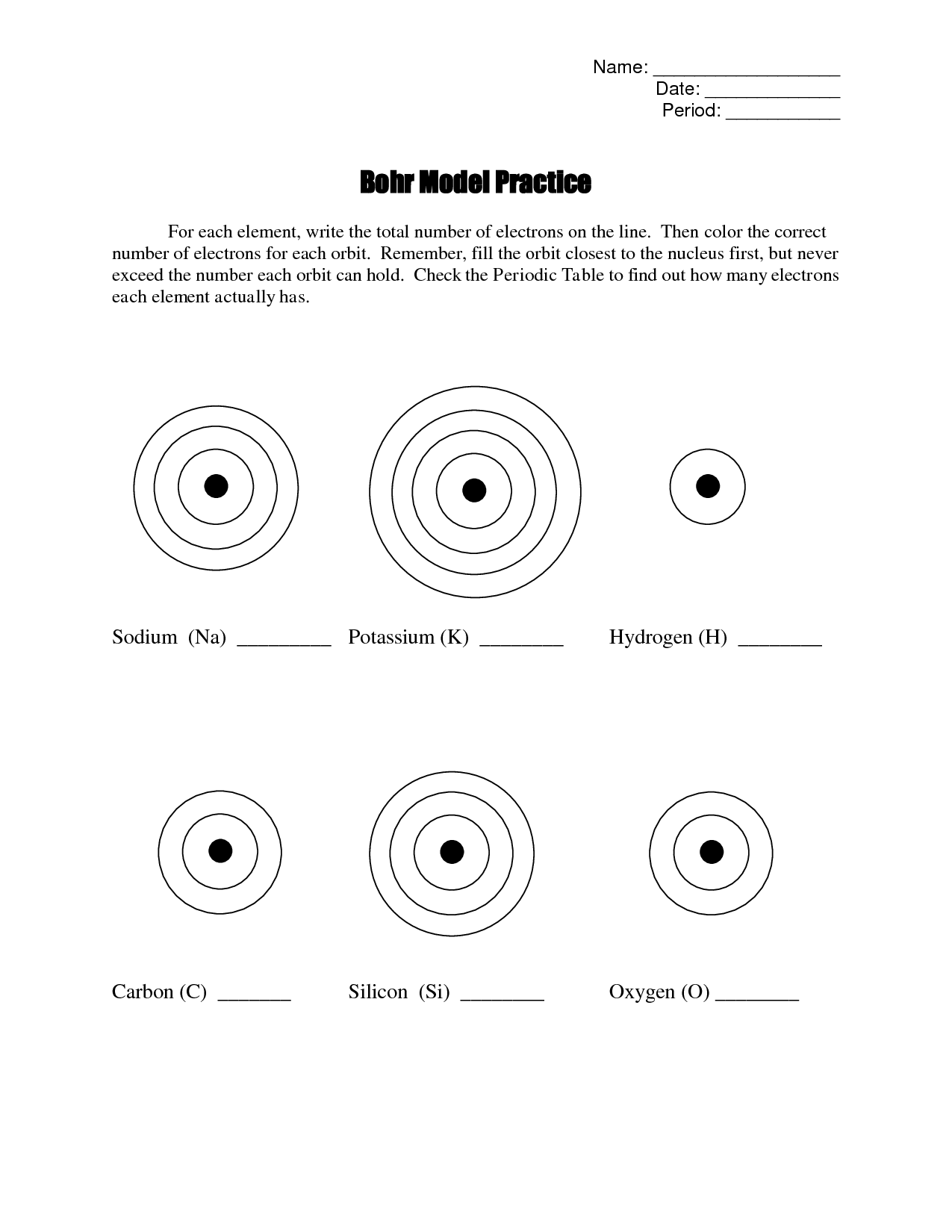
- Bohr Model Worksheet Answers
- How Do You Draw a Bohr Model
- Atomic Model Worksheet Answers
- Bohr Atomic Model Worksheet
- Models of the Atom Worksheet Answers
- Atom Particle Worksheet Answer
- Modern Atomic Theory Worksheet
- Atomic Structure Worksheet Answers
- Bohr Model Atomic Structure Worksheet Answers
- Theory and Atomic Structure Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
Who proposed the first atomic model?
The first atomic model was proposed by the English chemist John Dalton in the early 19th century. Dalton's atomic theory suggested that all matter is made up of tiny, indivisible particles called atoms, which have specific properties and combine in fixed ratios to form compounds.
What are the main components of the Bohr model?
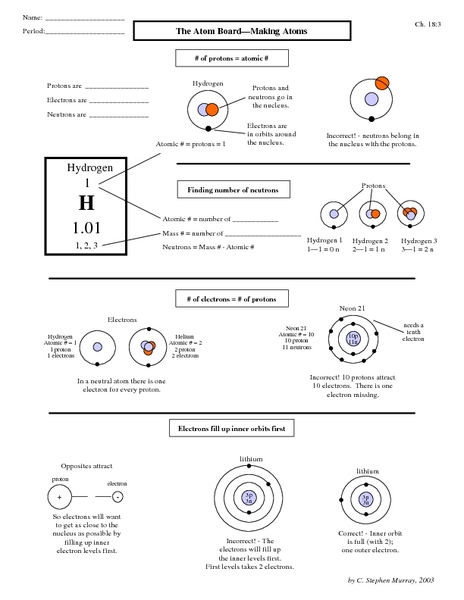
The main components of the Bohr model are the nucleus at the center, which contains protons and neutrons, and electron orbits that are represented as fixed paths around the nucleus. These orbits are quantized, meaning electrons can only occupy specific energy levels and transition between them by absorbing or emitting photons. The model also includes the concept of angular momentum quantization and the idea that electrons can jump between energy levels by emitting or absorbing photons of specific energies.
What distinguishes the Rutherford model from the previous atomic models?
The Rutherford model, proposed by Ernest Rutherford in 1911, distinguished itself from previous atomic models by introducing the concept of a dense, positively charged nucleus at the center of the atom, surrounded by orbiting electrons. This was a departure from earlier models, such as the Thomson and Dalton models, which did not account for the presence of a nucleus or the arrangement of electrons within the atom. Rutherford's model was based on his famous gold foil experiment, which demonstrated that most of the mass and positive charge of an atom is concentrated in a small, central nucleus.
What is the significance of the electron cloud in the modern atomic model?
The electron cloud is significant in the modern atomic model because it represents the probable location of electrons within an atom. This model describes electrons as existing in regions of space around the nucleus, rather than in fixed orbits. The electron cloud helps to explain the behavior of electrons and their involvement in chemical reactions, as it indicates the likelihood of finding an electron at a specific distance from the nucleus. This model is more accurate in describing the behavior of electrons in atoms compared to older models, such as the Bohr model.
What is the concept of energy levels in the atomic model?
In the atomic model, energy levels refer to the specific and discrete amount of energy that an electron can have within an atom. These energy levels are quantized, meaning that electrons can only exist at certain energy levels and not between them. When an electron absorbs or emits energy, it moves to a different energy level. The energy levels are represented by quantum numbers and are crucial in understanding the behavior of electrons in an atom.
How does the quantum mechanical model differ from previous atomic models?
The quantum mechanical model differs from previous atomic models, such as the Bohr model, by incorporating the principles of quantum mechanics to accurately describe the behavior of electrons in atoms. Unlike earlier models that depicted electrons as moving in fixed orbits around the nucleus, the quantum mechanical model represents electrons as existing in probability clouds or orbitals where their precise location and momentum cannot be determined simultaneously. Furthermore, the quantum mechanical model allows for a more comprehensive understanding of the wave-particle duality of electrons and provides a more sophisticated and accurate description of atomic phenomena.
What are orbitals in the atomic model?
Orbitals in the atomic model are regions of space around an atomic nucleus where electrons are most likely to be found. These regions have different shapes and sizes and can hold up to a specific number of electrons based on their energy levels. Orbitals help to describe the behavior and properties of electrons in an atom, including their distribution and energy levels.
How is the concept of electron spin incorporated in the atomic model?
The concept of electron spin is incorporated in the atomic model through the Pauli Exclusion Principle, which states that no two electrons can have the same set of quantum numbers. This principle takes into account the fact that electrons have intrinsic spin and are either spin up or spin down. In the atomic model, electron spin plays a crucial role in determining the allowed energy levels and configurations of electrons in atoms, giving rise to the unique properties of different elements on the periodic table.
What role does the nucleus play in the atomic model?
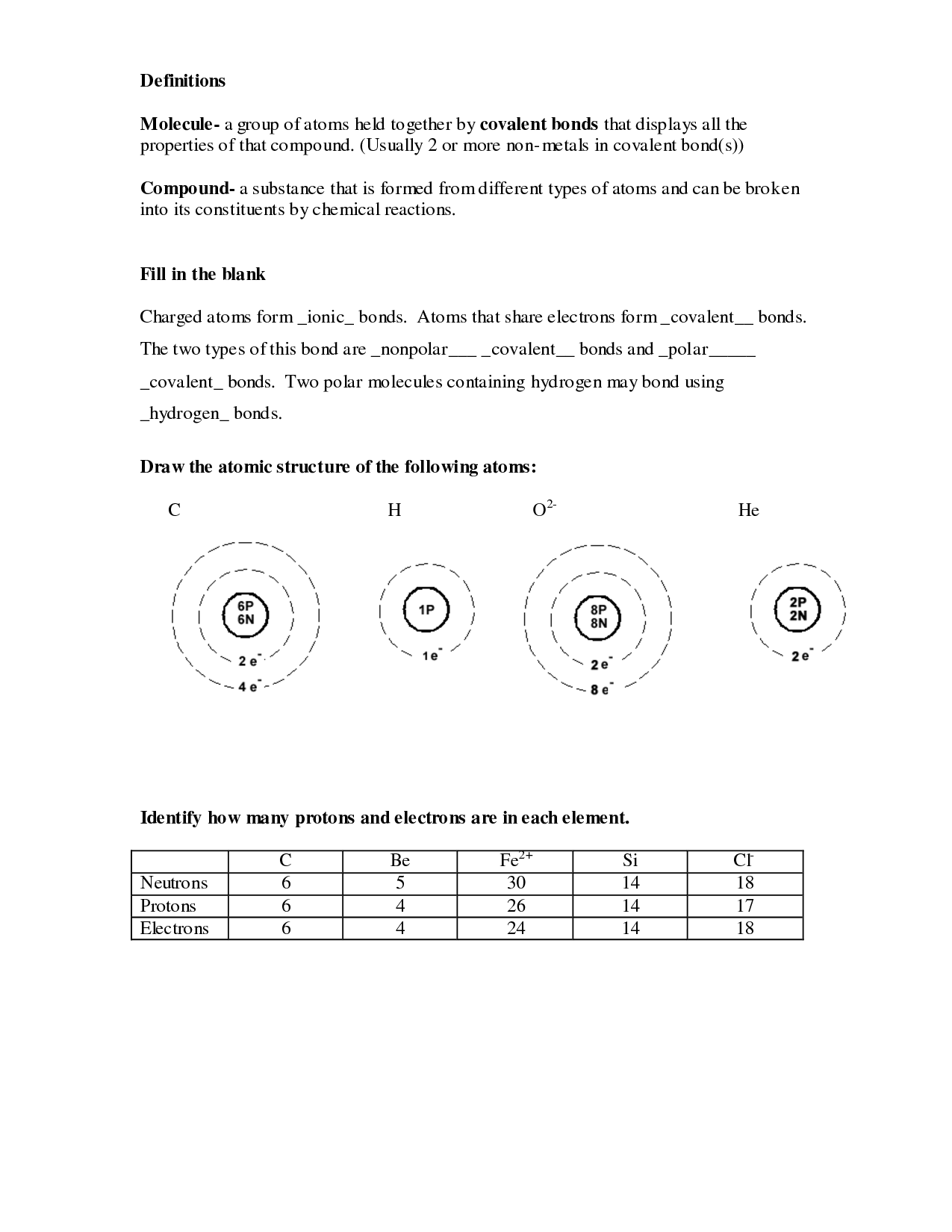
The nucleus is the central part of an atom that contains protons and neutrons. In the atomic model, the nucleus is responsible for providing the atom with its mass and holding together the positively charged protons with the neutral neutrons through the strong nuclear force. The electrons orbit around the nucleus in various energy levels, determining the chemical properties and bonding behavior of the atom.
How do atomic models contribute to our understanding of chemical reactions?
Atomic models contribute to our understanding of chemical reactions by providing a visual representation of how atoms interact and rearrange during a reaction. By depicting the arrangement of electrons, protons, and neutrons within an atom, these models help us understand the structure of different elements and how they combine to form compounds. They also illustrate how bonds form and break during chemical reactions, leading to a transformation of substances. By studying these models, scientists can predict the behavior of molecules, identify reaction mechanisms, and design new materials with specific properties, ultimately advancing our knowledge of chemistry.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments