Arrangement of Periodic Table Worksheet
The arrangement of the periodic table is a fundamental concept in chemistry that every student must understand. Whether you are a high school student preparing for an exam or a college student diving deeper into the subject, a well-designed worksheet can make all the difference. A comprehensive periodic table worksheet will ensure that you grasp the organization of elements, their respective properties, and their relationships to one another, allowing you to excel in your studies.
Table of Images 👆
- Printable Electron Configuration Periodic Table
- Electron Configuration Worksheet Answer Key
- Writing Ionic Compound Formula Worksheet Answers
- Electron Configuration Practice Worksheet
- Periodic Table Worksheets
- Chemistry Periodic Table Worksheet Answers
- Periodic Table Trends Worksheet
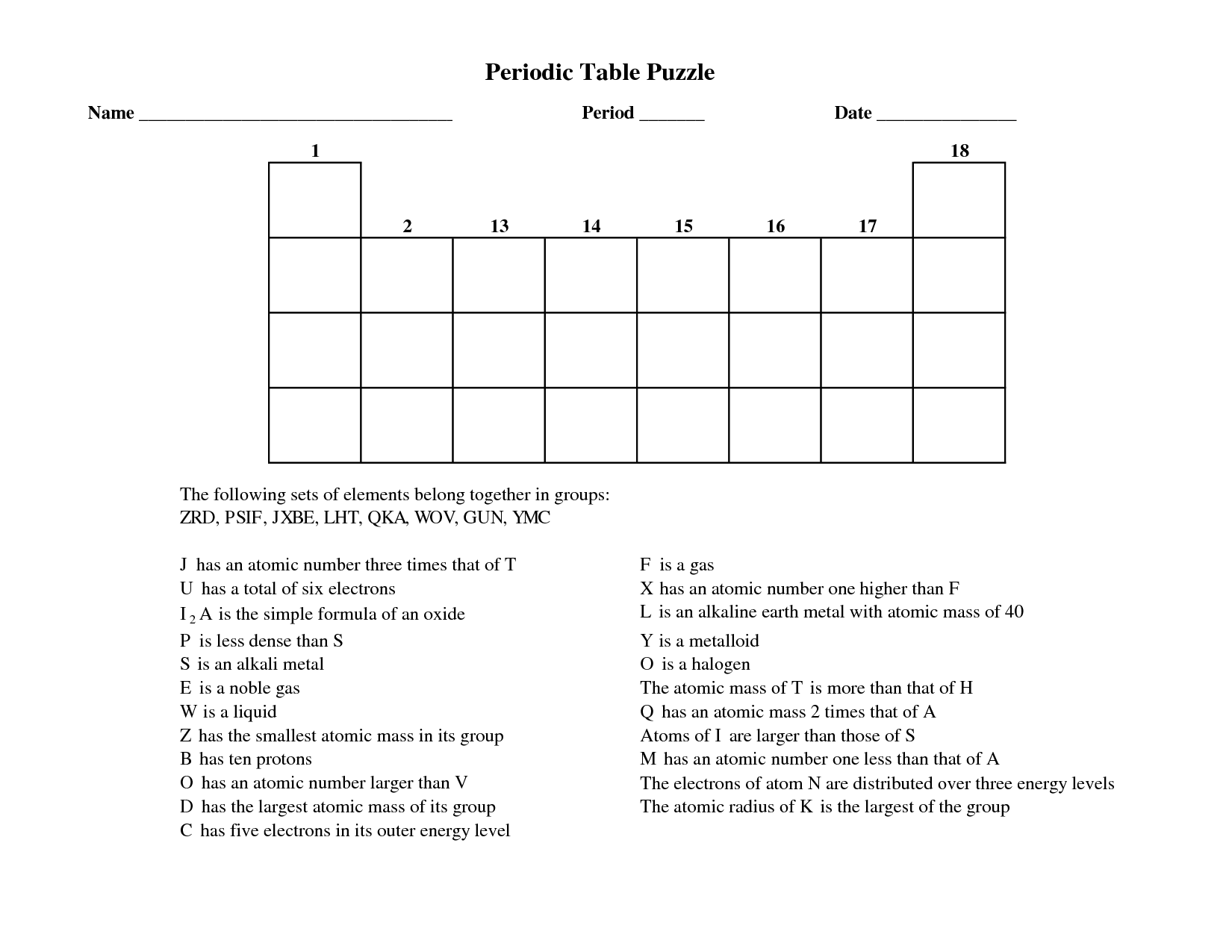
- Periodic Table Puzzle Worksheet Answers
- Periodic Table Scavenger Hunt Worksheet Answers
- Periodic Table Worksheet Answers
- Blank Periodic Table
- Periodic Table Coloring Worksheet
- Electron Arrangement in Atoms Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
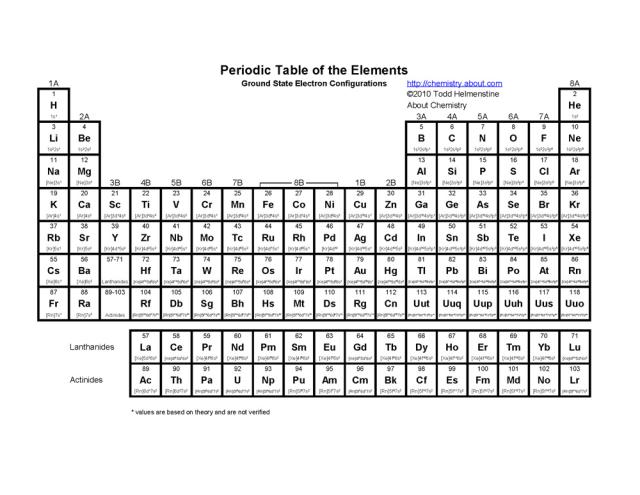
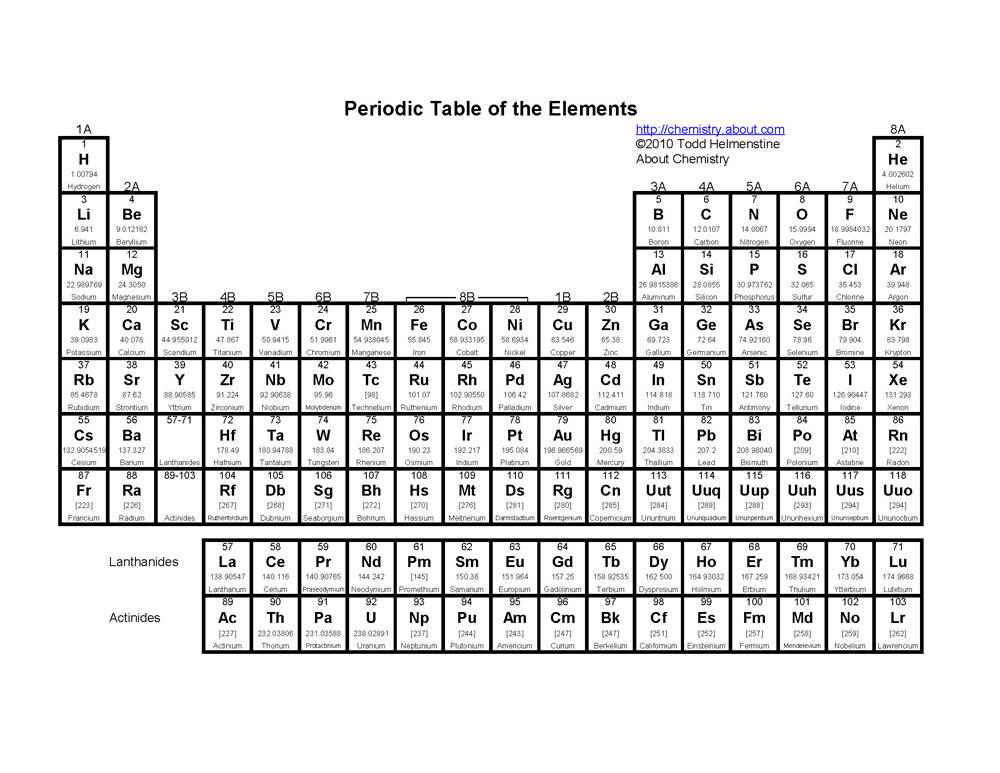
What is the arrangement of elements in the periodic table based on?
The arrangement of elements in the periodic table is based on their atomic number, which represents the number of protons in an atom's nucleus. This arrangement allows elements with similar chemical properties to be grouped together in columns or families, while elements with increasing atomic numbers are placed in rows or periods to show trends in their chemical and physical properties.
How many periods are there in the periodic table?
There are a total of 7 periods in the periodic table, which represent the rows in which elements are organized based on their increasing atomic number. Each period signifies a new energy level or electron shell for the elements within it.
How are elements organized within each period?
Elements within each period are organized based on their increasing atomic number, which reflects the number of protons in the nucleus of an atom. This atomic number correlates with the number of electrons in the atom, and as you move from left to right across a period, the number of protons and electrons increases by one with each successive element. This organizational structure allows for trends and patterns in the properties of elements to be observed as you move across a period.
What are groups in the periodic table?
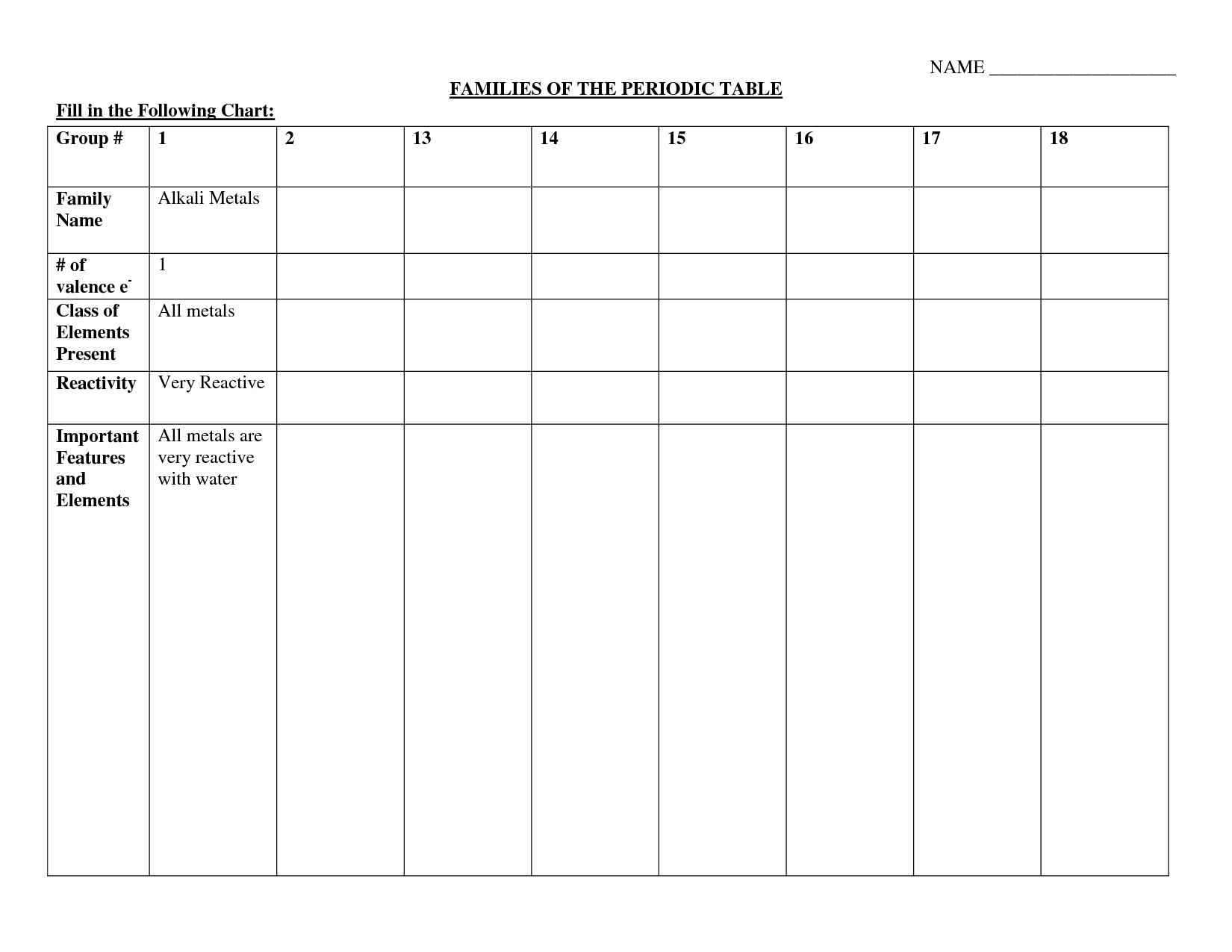
Groups in the periodic table are the columns of elements categorized based on similar chemical properties and valence electron configurations. There are total 18 groups in the periodic table, each denoted by a number or a letter, with each group having elements that share similar physical and chemical properties. Group 1 includes alkali metals, Group 2 includes alkaline earth metals, Group 17 includes halogens, and Group 18 includes noble gases, showcasing the pattern of elemental properties within each group.
How many groups are there in the periodic table?
There are 18 groups in the periodic table, each representing a different set of elements with similar chemical properties.
How are elements organized within each group?
Elements within each group are organized based on their atomic number, which represents the number of protons in the nucleus of an atom. Within each group, elements have similar chemical properties due to having the same number of valence electrons, which determines their reactivity. As you move down a group in the periodic table, the elements increase in atomic number and develop similar chemical behaviors, despite having different atomic masses and physical properties.
What are the main group elements?
The main group elements, also known as representative elements, are the elements in groups 1, 2, and 13 to 18 in the periodic table. These elements are characterized by their typical chemical properties and are found in the s-block and p-block of the periodic table. They include metals, metalloids, and nonmetals and are crucial for forming the diversity of compounds and materials found in nature.
How are transition elements different from main group elements?
Transition elements have partially filled d or f orbitals, characteristic of their position in the d-block of the periodic table, while main group elements have fully filled s and p orbitals, characteristic of their position in the s and p-blocks of the periodic table. Transition elements typically exhibit variable oxidation states and form colored compounds due to their partially filled d orbitals, while main group elements tend to form compounds in which the s and p orbitals are utilized for bonding. Additionally, transition elements often act as catalysts due to their ability to undergo redox reactions easily, whereas main group elements are less likely to exhibit catalytic behavior.
What are the characteristics of elements in the alkali metal group?
Elements in the alkali metal group have characteristics such as being highly reactive, having a single valence electron, low melting and boiling points, soft metals, exhibiting metallic bonding, and forming ionic compounds easily. They are typically found in Group 1 of the periodic table and include elements like lithium, sodium, potassium, rubidium, cesium, and francium.
What are the characteristics of elements in the halogen group?
Elements in the halogen group, such as fluorine, chlorine, bromine, iodine, and astatine, are highly reactive nonmetals with seven valence electrons. They have a tendency to gain one electron to achieve a stable octet configuration, making them strong oxidizing agents. Halogens exist in various states of matter at room temperature, and they exhibit distinct colors and strong odor. Additionally, they readily form compounds with metals and are often found in salts in nature.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments