And Specific Heat Capacity Worksheet
Are you a high school or college student looking to learn more about specific heat capacity? If so, you've come to the right place! In this blog post, we will be discussing worksheets that are specifically designed to help you understand and practice problems related to this important concept in thermodynamics. These worksheets will provide you with an organized and structured approach to learning about specific heat capacity and its various applications. So, let's dive in and explore the benefits of using a specific heat capacity worksheet!
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
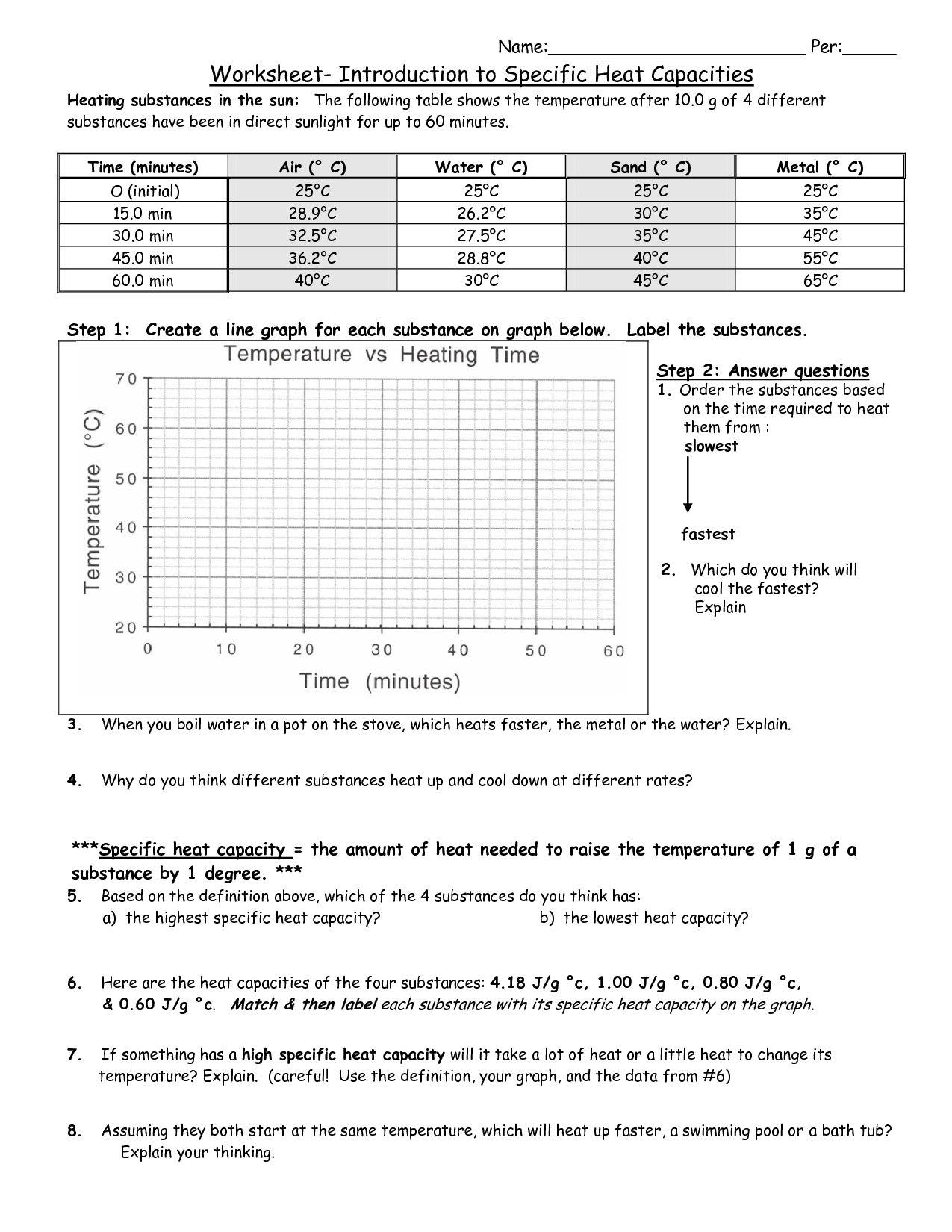
What is specific heat capacity?
Specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one degree Celsius (or one Kelvin). It is a characteristic property of a material and is expressed in units of J/kg°C (joules per kilogram per degree Celsius).
How is specific heat capacity measured?
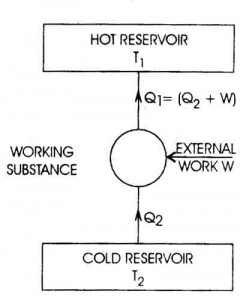
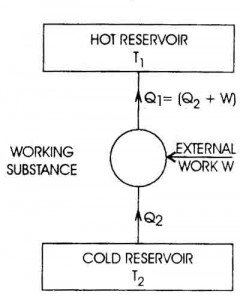
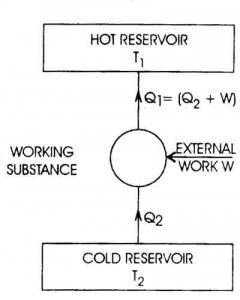
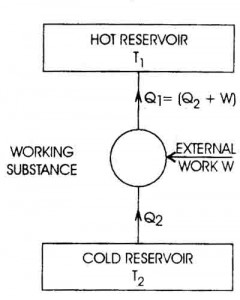
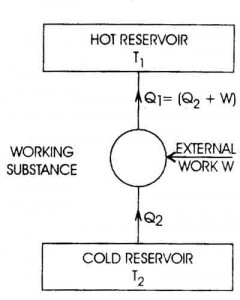
Specific heat capacity is typically measured using a calorimeter, a device designed to measure the amount of heat absorbed or released during a physical or chemical process. The sample with a known mass is heated or cooled to a specific temperature, then placed in the calorimeter where its temperature change is measured. By knowing the amount of heat applied or removed and the mass and temperature change of the sample, the specific heat capacity can be calculated using the formula Q = mc?T, where Q is the heat energy, m is the mass of the sample, c is the specific heat capacity, and ?T is the temperature change.
What are some factors that affect the specific heat capacity of a substance?
The specific heat capacity of a substance is affected by factors such as the molecular structure of the substance, the presence of impurities or alloys, the temperature and pressure of the substance, and the phase or state of matter (solid, liquid, or gas). Additionally, the specific heat capacity can be influenced by the mass and density of the substance, as well as any phase transitions or chemical reactions that occur.
Define the equation for calculating heat transfer using specific heat capacity.
The equation for calculating heat transfer using specific heat capacity is Q = mc?T, where Q represents the amount of heat transferred, m is the mass of the substance, c is the specific heat capacity of the substance, and ?T is the temperature change. This equation quantifies the amount of heat transferred when a substance undergoes a change in temperature.
How does specific heat capacity relate to temperature change?
The specific heat capacity of a substance is the amount of energy required to change the temperature of one unit mass of that substance by one degree Celsius. Therefore, a substance with a high specific heat capacity requires more energy to raise its temperature compared to a substance with a lower specific heat capacity. This means that substances with higher specific heat capacities will experience smaller temperature changes for a given amount of heat added or removed compared to substances with lower specific heat capacities.
Give an example of a substance with a high specific heat capacity.
Water is an example of a substance with a high specific heat capacity. It has a specific heat capacity of 4.18 J/g°C, which means it can absorb or release a significant amount of heat energy without undergo a large temperature change. This property of water is why it takes a long time for large bodies of water, such as oceans, to heat up or cool down, ultimately influencing climate patterns and regulating temperature on Earth.
Explain why water has a high specific heat capacity.
Water has a high specific heat capacity due to its strong hydrogen bonding. These hydrogen bonds in water allow for a high amount of energy to be absorbed without a significant increase in temperature. This means that water can absorb and release large amounts of heat before its temperature changes significantly, making it an effective regulator of temperature in both living organisms and the environment.
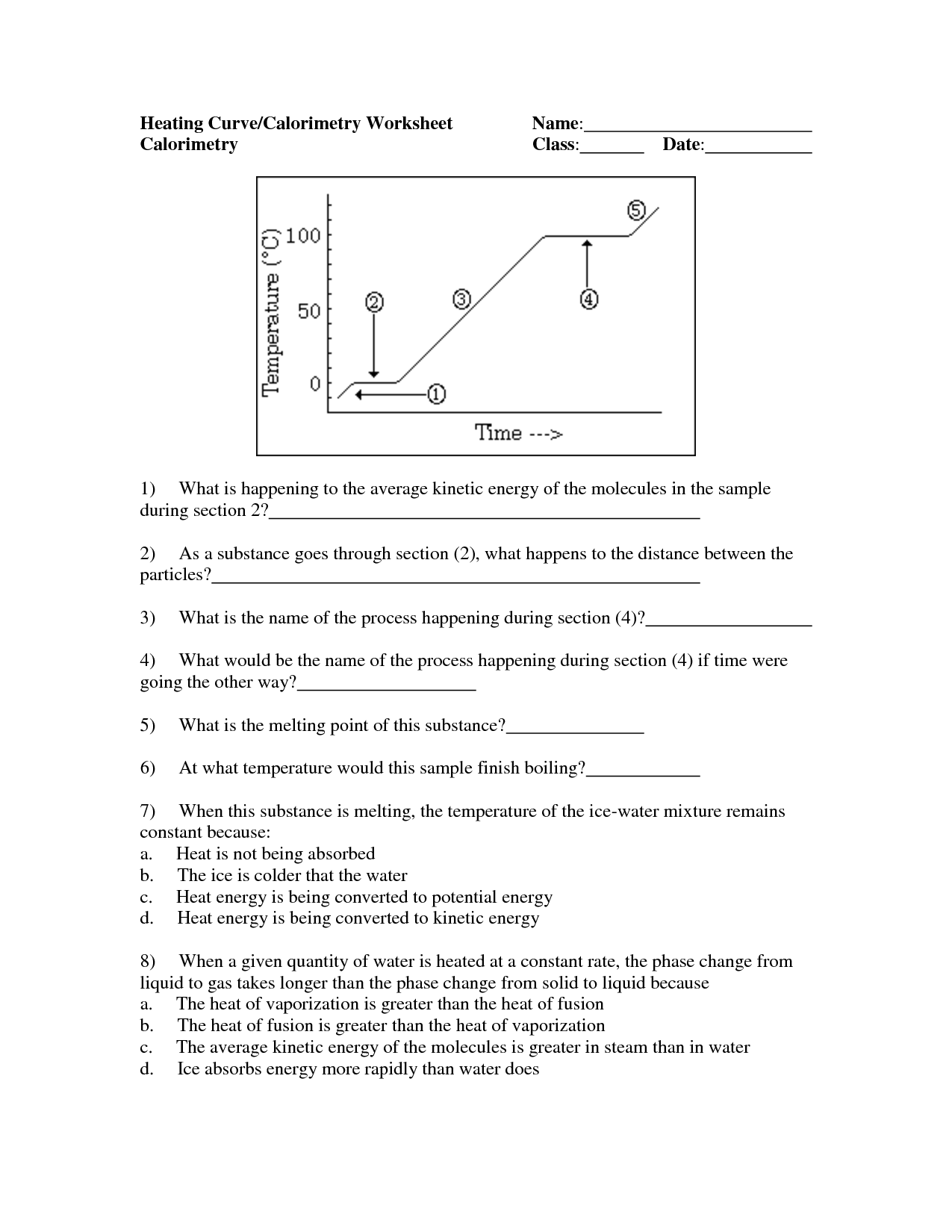
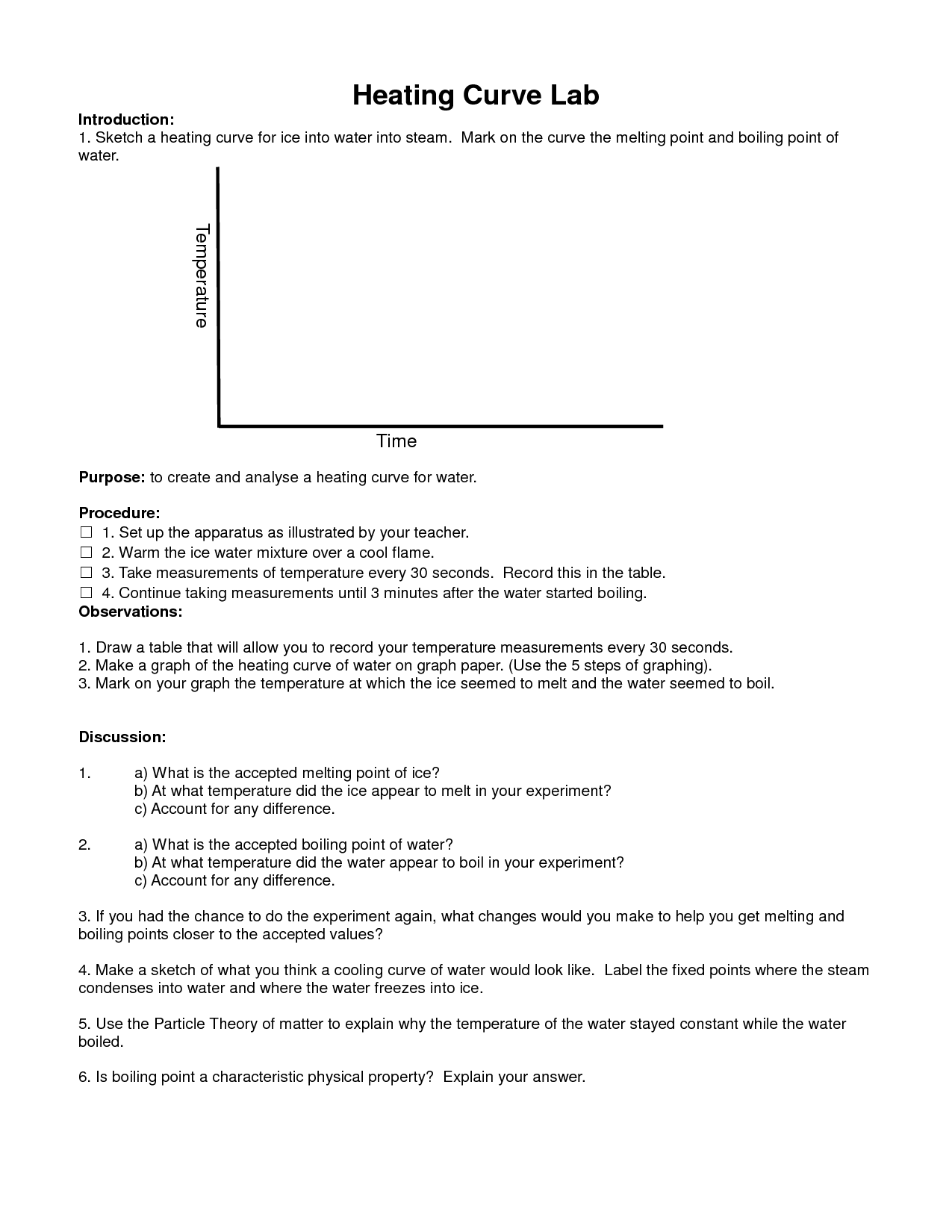
How does specific heat capacity affect the boiling and melting points of a substance?
The specific heat capacity of a substance affects its boiling and melting points by determining the amount of energy required to change its phase. A substance with a higher specific heat capacity will require more energy to reach the boiling or melting point, resulting in a higher boiling and melting point. Conversely, a substance with a lower specific heat capacity will require less energy to reach these points, resulting in a lower boiling and melting point. Essentially, the specific heat capacity influences the temperature at which a substance transitions from one phase to another.
Why is it important to consider specific heat capacity in thermodynamic calculations?
Specific heat capacity is important in thermodynamic calculations because it quantifies the amount of heat energy required to change the temperature of a substance. By taking into account the specific heat capacity of a material, one can accurately determine the amount of energy needed to achieve a desired change in temperature during processes such as heating or cooling. This parameter plays a crucial role in understanding and predicting the behavior of systems in thermodynamic processes, ensuring accurate calculations and efficient energy utilization.
How can specific heat capacity be determined experimentally?
Specific heat capacity can be determined experimentally by measuring the amount of heat energy transferred to a material to change its temperature. This can be done by using a calorimeter to heat a known mass of the substance with a known amount of energy while measuring the temperature change. By equating the heat energy supplied to the substance with the mass, temperature change, and specific heat capacity of the material, the specific heat capacity can be calculated using the formula q = mc?T, where q is the heat energy, m is the mass of the substance, c is the specific heat capacity, and ?T is the temperature change.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments