4th Grade Science Worksheets Density
Are you in search of engaging and educational resources to support your 4th-grade student's learning? Look no further! Our 4th-grade science worksheets on density are specifically designed to enhance their understanding of this fundamental scientific concept. With captivating visuals and thought-provoking questions, these worksheets are sure to keep your child engaged while learning about density.
Table of Images 👆
- Fifth Grade Science Worksheets
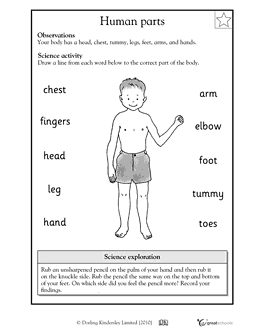
- Human Body Parts Worksheet for 2nd Grade
- 8th Grade Earth Science Worksheets
- 2nd Grade Solids Liquids and Gases Worksheets
- Physical Science Newtons Laws Worksheet Answer Key
- Plate Tectonics Worksheet Answer Key
- Density Jokes Science
- Chemistry Atomic Structure Worksheet Answers
More 4th Grade Worksheets
4th Grade Elapsed Time WorksheetsIrregular Plural Worksheets 4th Grade
Rotational Symmetry Worksheets 4th Grade
Simple Circuit Worksheets 4th Grade
Long Division with Remainders Worksheets 4th Grade
Fourth Grade Reading Comp Worksheets
Reading Response Worksheets 4th Grade
4th Grade Essay Writing Worksheets
Worksheets 4th Grade Narrative Writing
Long Lined Paper Worksheets 4th Grade Essay-Writing
What is density?
Density is a measure of how much mass is contained in a given volume. It is calculated by dividing an object's mass by its volume and is typically expressed in units such as grams per cubic centimeter or kilograms per cubic meter. Materials with a high density have more mass packed into a small volume, while materials with a low density have less mass distributed over a larger volume.
How is density calculated?
Density is calculated by dividing the mass of an object by its volume. The formula for density is Density = Mass/Volume. Mass is typically measured in grams or kilograms, while volume is measured in cubic centimeters or cubic meters. By measuring the mass and volume of an object, you can determine its density, which is a measure of how much mass is contained in a given volume of the substance.
What are the units of density?
Density is typically expressed in units of mass per unit volume, such as grams per cubic centimeter (g/cm^3) or kilograms per cubic meter (kg/m^3).
What is the relationship between density and mass?
Density and mass are related in that density is the mass of an object divided by its volume. This means that as the mass of an object increases while its volume remains constant, the density of the object also increases. Conversely, if the mass of an object decreases while the volume stays the same, the density of the object decreases. This relationship demonstrates how mass and density are intertwined attributes that can impact each other based on the size and composition of the object in question.
What is the relationship between density and volume?
Density is the amount of mass of a substance per unit volume. So, the relationship between density and volume is that density is inversely proportional to volume. This means that as the volume of a substance increases, the density decreases, and vice versa.
How does the density of an object determine if it will sink or float in a fluid?
The density of an object determines if it will sink or float in a fluid based on the principle of buoyancy. An object will float if its density is less than the density of the fluid it is placed in, because the buoyant force exerted by the fluid is greater than the force of gravity acting on the object. Conversely, an object will sink if its density is greater than the density of the fluid, as the buoyant force will be insufficient to counteract the force of gravity pulling the object downward.
How can you determine the density of an irregularly shaped object?
To determine the density of an irregularly shaped object, you can follow these steps: First, measure the object's mass using a scale. Next, fill a graduated cylinder with water and record the initial volume. Submerge the object in the water and measure the new volume. Finally, calculate the density by dividing the mass of the object by the change in volume. Density equals mass divided by volume.
What are some examples of materials with high density?
Some examples of materials with high density include lead, gold, uranium, platinum, tungsten, osmium, and iridium. These materials are all very dense due to their atomic structure and heavy atomic weight, resulting in a high mass per unit volume.
What are some examples of materials with low density?
Some examples of materials with low density include cork, balsa wood, styrofoam, aerogel, and helium gas. These materials are known for being lightweight and having low mass compared to their volume, making them ideal for applications where weight reduction is important.
How does temperature affect the density of a substance?
Temperature affects the density of a substance by influencing the kinetic energy of its particles. As temperature increases, the particles within the substance move faster and further apart, causing the substance to expand and become less dense. Conversely, as temperature decreases, the particles slow down and come closer together, leading to the substance contracting and becoming more dense.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments